Quinquenoside R1CAS# 85013-02-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85013-02-1 | SDF | Download SDF |
| PubChem ID | 101679657.0 | Appearance | Powder |
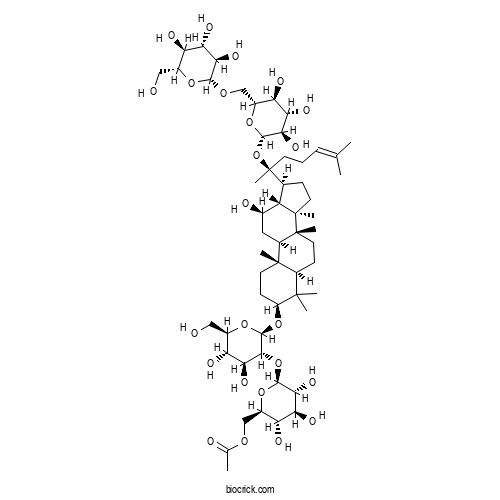
| Formula | C56H94O24 | M.Wt | 1151.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3S,4S,5R,6S)-6-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxyhept-5-en-2-yl]-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]oxan-3-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl acetate | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)COC(=O)C)O)O)O)C)C)O)C)OC7C(C(C(C(O7)COC8C(C(C(C(O8)CO)O)O)O)O)O)O)C | ||
| Standard InChIKey | SNHCPECPLQRJNL-GWNBYJSOSA-N | ||
| Standard InChI | InChI=1S/C56H94O24/c1-24(2)11-10-15-56(9,80-50-46(71)42(67)39(64)31(77-50)23-73-48-44(69)40(65)36(61)28(20-57)74-48)26-12-17-55(8)35(26)27(60)19-33-53(6)16-14-34(52(4,5)32(53)13-18-54(33,55)7)78-51-47(43(68)37(62)29(21-58)75-51)79-49-45(70)41(66)38(63)30(76-49)22-72-25(3)59/h11,26-51,57-58,60-71H,10,12-23H2,1-9H3/t26-,27+,28+,29+,30+,31+,32-,33+,34-,35-,36+,37+,38+,39+,40-,41-,42-,43-,44+,45+,46+,47+,48+,49-,50-,51-,53-,54+,55+,56-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Quinquenoside R1 Dilution Calculator

Quinquenoside R1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8686 mL | 4.3428 mL | 8.6856 mL | 17.3712 mL | 21.714 mL |
| 5 mM | 0.1737 mL | 0.8686 mL | 1.7371 mL | 3.4742 mL | 4.3428 mL |
| 10 mM | 0.0869 mL | 0.4343 mL | 0.8686 mL | 1.7371 mL | 2.1714 mL |
| 50 mM | 0.0174 mL | 0.0869 mL | 0.1737 mL | 0.3474 mL | 0.4343 mL |
| 100 mM | 0.0087 mL | 0.0434 mL | 0.0869 mL | 0.1737 mL | 0.2171 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-hydroxyl emodin-1-methyl ether
Catalog No.:BCX0683
CAS No.:346434-45-5
- Maceneolignan H
Catalog No.:BCX0682
CAS No.:1314042-85-7
- 1-Methyl Emodin
Catalog No.:BCX0681
CAS No.:3775-08-4
- Polyporusterone B
Catalog No.:BCX0680
CAS No.:141360-89-6
- Emodin anthrone
Catalog No.:BCX0679
CAS No.:491-60-1
- Anhydrosafflor yellow B
Catalog No.:BCX0678
CAS No.:184840-84-4
- Cis-Emodin bianthrone
Catalog No.:BCX0677
CAS No.:61281-19-4
- Polyporusterone A
Catalog No.:BCX0676
CAS No.:141360-88-5
- Dendronobilin B
Catalog No.:BCX0675
CAS No.:1002717-96-5
- 5,7-Dihydroxycoumarin
Catalog No.:BCX0674
CAS No.:2732-18-5
- Urolithin A
Catalog No.:BCX0673
CAS No.:1143-70-0
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
- Sequoyitol
Catalog No.:BCX0685
CAS No.:523-92-2
- Cassiaside C2
Catalog No.:BCX0686
CAS No.:1958039-40-1
- 3-Hydroxy-1,2-dimethoxy-anthraquinone
Catalog No.:BCX0687
CAS No.:10383-62-7
- Gentianose
Catalog No.:BCX0688
CAS No.:25954-44-3
- Koumidine
Catalog No.:BCX0689
CAS No.:1358-75-4
- Farnesene
Catalog No.:BCX0690
CAS No.:502-61-4
- 8,9-epoxy-3,10-diisobutyryloxythymol
Catalog No.:BCX0691
CAS No.:22518-06-5
- N-(3-methoxybenzyl)-octadecanamide
Catalog No.:BCX0692
CAS No.:1429659-99-3
- N-benzyl-heptadecanamide
Catalog No.:BCX0693
CAS No.:883715-19-3
- Polygalasaponin XXVIII
Catalog No.:BCX0694
CAS No.:176182-01-7
- Cavidine
Catalog No.:BCX0695
CAS No.:32728-75-9
- Quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside]
Catalog No.:BCX0696
CAS No.:83048-35-5
Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy.[Pubmed:27746686]
J Ginseng Res. 2016 Oct;40(4):344-350.
BACKGROUND: Mountain-cultivated ginseng (MCG) and cultivated ginseng (CG) both belong to Panax ginseng and have similar ingredients. However, their pharmacological activities are different due to their significantly different growth environments. METHODS: An ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS)-based approach was developed to distinguish MCG and CG. Multivariate statistical methods, such as principal component analysis and supervised orthogonal partial-least-squares discrimination analysis were used to select the influential components. RESULTS: Under optimized UPLC-QTOF-MS/MS conditions, 40 ginsenosides in both MCG and CG were unambiguously identified and tentatively assigned. The results showed that the characteristic components of CG and MCG included ginsenoside Ra3/isomer, gypenoside XVII, Quinquenoside R1, ginsenoside Ra7, notoginsenoside Fe, ginsenoside Ra2, ginsenoside Rs6/Rs7, malonyl ginsenoside Rc, malonyl ginsenoside Rb1, malonyl ginsenoside Rb2, palmitoleic acid, and ethyl linoleate. The malony ginsenosides are abundant in CG, but higher levels of the minor ginsenosides were detected in MCG. CONCLUSION: This is the first time that the differences between CG and MCG have been observed systematically at the chemical level. Our results suggested that using the identified characteristic components as chemical markers to identify different ginseng products is effective and viable.
[Chemical constituents of leaves of Panax japonicus var. major].[Pubmed:25095375]
Zhongguo Zhong Yao Za Zhi. 2014 May;39(9):1635-8.
Seven compounds were isolated from the leaves of Panax japonicus var. major by chromatographic methods including silica gel, Sephadex LH-20, ODS and semi-preparative HPLC. Their structures were elucidated by their physical and chemical properties and spectral data analysis as 5, 7-dihydroxy-8-methoxyl flavone (1), ginsenoside Rs2 (2), Quinquenoside R1 (3), ginsenoside Rs1 (4), notoginsenoside Fe (5), ginsenoside Rd2 (6) and gypenosiden IX (7). Among them, compound 1 was obtained from the Panax genus for the first time, and compounds 2-7 were isolated from this plant for the first time.


