5,7-DihydroxycoumarinCAS# 2732-18-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2732-18-5 | SDF | Download SDF |
| PubChem ID | 5324654.0 | Appearance | Powder |
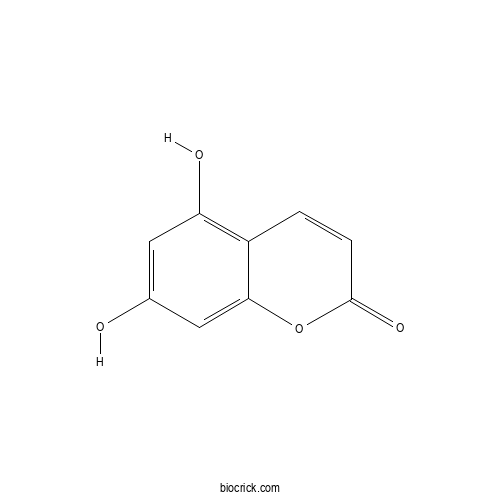
| Formula | C9H6O4 | M.Wt | 178.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,7-dihydroxychromen-2-one | ||
| SMILES | C1=CC(=O)OC2=CC(=CC(=C21)O)O | ||
| Standard InChIKey | KIQQFVJHWNCGAU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H6O4/c10-5-3-7(11)6-1-2-9(12)13-8(6)4-5/h1-4,10-11H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5,7-Dihydroxycoumarin Dilution Calculator

5,7-Dihydroxycoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6136 mL | 28.0678 mL | 56.1356 mL | 112.2712 mL | 140.3391 mL |
| 5 mM | 1.1227 mL | 5.6136 mL | 11.2271 mL | 22.4542 mL | 28.0678 mL |
| 10 mM | 0.5614 mL | 2.8068 mL | 5.6136 mL | 11.2271 mL | 14.0339 mL |
| 50 mM | 0.1123 mL | 0.5614 mL | 1.1227 mL | 2.2454 mL | 2.8068 mL |
| 100 mM | 0.0561 mL | 0.2807 mL | 0.5614 mL | 1.1227 mL | 1.4034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Urolithin A
Catalog No.:BCX0673
CAS No.:1143-70-0
- Sorbitol
Catalog No.:BCX0672
CAS No.:50-70-4
- Ergolide
Catalog No.:BCX0671
CAS No.:54999-07-4
- Ankaflavin
Catalog No.:BCX0670
CAS No.:50980-32-0
- Bigelovin
Catalog No.:BCX0669
CAS No.:3668-14-2
- Trans-Emodin bianthrone
Catalog No.:BCX0668
CAS No.:61281-20-7
- Apigenin-7-diglucuronide
Catalog No.:BCX0667
CAS No.:74696-01-8
- Safranal
Catalog No.:BCX0666
CAS No.:116-26-7
- N-benzylpentadecanamide
Catalog No.:BCX0665
CAS No.:1572037-13-8
- Isotoosendanin
Catalog No.:BCX0664
CAS No.:97871-44-8
- 6''-O-apiosyl-Visammioside
Catalog No.:BCX0663
CAS No.:2254096-97-2
- Ophiopogonside A
Catalog No.:BCX0662
CAS No.:2423917-90-0
- Dendronobilin B
Catalog No.:BCX0675
CAS No.:1002717-96-5
- Polyporusterone A
Catalog No.:BCX0676
CAS No.:141360-88-5
- Cis-Emodin bianthrone
Catalog No.:BCX0677
CAS No.:61281-19-4
- Anhydrosafflor yellow B
Catalog No.:BCX0678
CAS No.:184840-84-4
- Emodin anthrone
Catalog No.:BCX0679
CAS No.:491-60-1
- Polyporusterone B
Catalog No.:BCX0680
CAS No.:141360-89-6
- 1-Methyl Emodin
Catalog No.:BCX0681
CAS No.:3775-08-4
- Maceneolignan H
Catalog No.:BCX0682
CAS No.:1314042-85-7
- 2-hydroxyl emodin-1-methyl ether
Catalog No.:BCX0683
CAS No.:346434-45-5
- Quinquenoside R1
Catalog No.:BCX0684
CAS No.:85013-02-1
- Sequoyitol
Catalog No.:BCX0685
CAS No.:523-92-2
- Cassiaside C2
Catalog No.:BCX0686
CAS No.:1958039-40-1
Identification of natural xanthine oxidase inhibitors: Virtual screening, anti-xanthine oxidase activity, and interaction mechanism.[Pubmed:38216015]
Int J Biol Macromol. 2024 Feb;259(Pt 2):129286.
Xanthine oxidase (XO) is a crucial target for hyperuricemia treatment(s). Naturally occurred XO inhibitors with minimal toxicity and high efficacy have attracted researchers' attention. With the goal of quickly identifying natural XO inhibitors, an integrated computational screening strategy was constructed by molecular docking and calculating the free energy of binding. Twenty-seven hits were achieved from a database containing 19,377 natural molecules. This includes fourteen known XO inhibitors and four firstly-reported inhibitors (isolicoflavonol, 5,7-Dihydroxycoumarin, parvifolol D and clauszoline M, IC(50) < 40 muM). Iolicoflavonol (hit 8, IC(50) = 8.45 +/- 0.68 muM) and 5,7-Dihydroxycoumarin (hit 25, IC(50) = 10.91 +/- 0.71 muM) displayed the great potency as mixed-type inhibitors. Docking study and molecular dynamics simulation revealed that both hits could interact with XO's primarily active site residues ARG880, MOS1328, and ASN768 of XO. Fluorescence spectroscopy studies showed that hit 8 bound to the active cavity region of XO, causing changes in XO's conformation and hydrophobicity. Hits 8 and 25 exhibit favorable Absorption, Distribution, Metabolism, and Excretion (ADME) properties. Additionally, no cytotoxicity against human liver cells was observed at their median inhibition concentrations against XO. Therefore, the present study offers isolicoflavonol and 5,7-Dihydroxycoumarin with the potential to be disease-modifying agents for hyperuricemia.
Design of 3-Phenylcoumarins and 3-Thienylcoumarins as Potent Xanthine Oxidase Inhibitors: Synthesis, Biological Evaluation, and Docking Studies.[Pubmed:37801332]
ChemMedChem. 2023 Nov 2;18(21):e202300400.
Coumarin scaffold has proven to be promising in the development of bioactive agents, such as xanthine oxidase (XO) inhibitors. Novel hydroxylated 3-arylcoumarins were designed, synthesized, and evaluated for their XO inhibition and antioxidant properties. 3-(3'-Bromophenyl)-5,7-Dihydroxycoumarin (compound 11) proved to be the most potent XO inhibitor, with an IC(50) of 91 nM, being 162 times better than allopurinol, one of the reference controls. Kinetic analysis of compound 11 and compound 5 [3-(4'-bromothien-2'-yl)-5,7-Dihydroxycoumarin], the second-best compound within the series (IC(50) of 280 nM), has been performed, and both compounds showed a mixed-type inhibition. Both compounds present good antioxidant activity (ability to scavenge ABTS radical) and are able to reduce reactive oxygen species (ROS) levels in H(2) O(2) -treated cells. In addition, they proved to be non-cytotoxic in a Caco-2 cells viability assay. Molecular docking studies have been carried out to correlate the compounds' theoretical and experimental binding affinity to the XO binding pocket.
Hydroxy-3-Phenylcoumarins as Multitarget Compounds for Skin Aging Diseases: Synthesis, Molecular Docking and Tyrosinase, Elastase, Collagenase and Hyaluronidase Inhibition, and Sun Protection Factor.[Pubmed:36296507]
Molecules. 2022 Oct 15;27(20):6914.
Skin aging is a progressive biological process of the human body, and it is not only time-dependent. Differently substituted 3-phenylcoumarins proved to efficiently inhibit tyrosinase. In the current work, new substitution patterns have been explored, and the biological studies were extended to other important enzymes involved in the processes of skin aging, as elastase, collagenase and hyaluronidase. From the studied series, five compounds presented inhibitory activity against tyrosinase, one compound against elastase, eight compounds against collagenase and two compounds against hyaluronidase, being five compounds dual inhibitors. The 3-(4'-Bromophenyl)-5,7-Dihydroxycoumarin (1) and 3-(3'-bromophenyl)-5,7-Dihydroxycoumarin (2) presented the best profiles against tyrosinase (IC(50) = 1.05 microM and 7.03 microM) and collagenase (IC(50) = 123.4 microM and 110.4 microM); the 3-(4'-bromophenyl)-6,7-dihydroxycoumarin (4) presented a good inhibition against tyrosinase and hyaluronidase; the 3-(3'-bromophenyl)-6,7-dihydroxycoumarin (5) showed an effective tyrosinase and elastase inhibition; and 6,7-dihydroxy-3-(3'-hydroxyphenyl)coumarin (11) presented a dual profile inhibition against collagenase and hyaluronidase. Furthermore, considering the overall activities tested, compounds 1 and 2 proved to be the most promising anti-aging compounds. These compounds also showed to have a photo-protective effect, without being cytotoxic to human skin keratinocyte cells. To predict the binding site with the target enzymes, computational studies were also carried out.
Forming coumarin C-glycosides via biocatalysis: Characterization of a C-glycosyltransferase from Angelica decursiva.[Pubmed:35569380]
Biochem Biophys Res Commun. 2022 Jul 23;614:85-91.
A glycosyl transferase, isolated from Angelica decursiva a medical herb rich in coumarin, shows C-glycosyl transferase activity by in vitro activity assay using 5,7-Dihydroxycoumarin as substrate, producing a C-glycosylated product at position C'8 along with the main product at C'6 position. Catalytic promiscuity assay shows that AdCGT also displays O- or C-glycosylation activity to other coumarins and flavonoids. When phloretin and 2,4,6-trihydroxyacetophenone were fed as substrates, AdCGT catalyzed the formation of di-C-glycosides. Therefore, AdCGT is a multifunctional glycosyltransferase with a broad substrate acceptability. This work highlights the potential of AdCGT as a catalyst for glycosylation of coumarin and reveals a new regio-selective C-glycosyltransferase, providing a basis for exploring the mechanism of coumarin glycosylation.
Two new derivatives of 8-prenyl-5,7-dihydroxycoumarin from the stems of Streblus ilicifolius (S.Vidal) Corn.[Pubmed:33939585]
Nat Prod Res. 2022 Oct;36(19):4967-4972.
From the EtOAc-soluble extract of the stems of Streblus ilicifolius (Moraceae), two new secondary metabolites named strebluses A (1) and B (2) were isolated. Their chemical structures have been concluded based on the chemical derivatisation and the spectroscopic interpretation. All compounds have been tested for their tyrosinase inhibitory activity. They showed weaker inhibitory activity than that of kojic acid (IC(50), 44.6 microM).[Formula: see text].
[Study on non-flavonoids chemical constituents from Spatholobi Caulis].[Pubmed:32237455]
Zhongguo Zhong Yao Za Zhi. 2020 Mar;45(5):1120-1127.
To study the non-flavonoids chemical constituents in water extract of Spatholobi Caulis. Some purification and analysis techniques like silica gel, D101-macroporous adsorptive resins, and Sephadex LH-20 column chromatographies as well as reversed phase high-performance liquid chromatography were used to isolate and analyze the phenolic acid esters and other type compounds from Spatholobi Caulis integrally. The structures of these compounds were identified by spectroscopic techniques such as nuclear magnetic resonance and high resolution mass spectrometries. Twenty-seven compounds, including phenolic acid, coumarin, lignan, terpene, alkaloid, and steroid compounds, were isolated from ethyl acetate and n-butanol fractions in water extract of Spatholobi Caulis, and they were identified as beta-sitosterol(1), feruli acid methyl ester(2), syringaresinol(3),(+)-medioresinol(4),(+)-epipinoresinol(5), p-acetylphenol(6), bolusanthin Ⅳ(7), evofolin B(8), salicylic acid(9), trans-p-hydroxy-cinnamic acid(10), abscisic acid(11), m-hydroxyphenol(12), C-veratroylglycol(13), p-hydroquinone(14), 8,9-dihydroxymegastigma-4,6-dien-3-one(15), p-hydroxybenzoic acid(16), 6,9-dihydroxymegastigma-4,7-dien-3-one(17), protocatechuic acid(18), protocatechuic acid methyl ester(19), 5,7-Dihydroxycoumarin(20), isolariciresinol(21), nicotinic acid(22), daucosterol(23),(+)-pinoresinol(24), stigmasterol(25), allantoin(26) and koaburaside(27), respectively. Furthermore, compounds 2-15, 19-22, 24 and 26 were isolated from genus Spatholobus for the first time.
Efficient synthesis of 9,10-dihydropyrano[2,3-h]chromene-2,8-dione derivatives in ionic liquid and the study of their antioxidant activity.[Pubmed:27080044]
Nat Prod Res. 2017 Jan;31(1):1-6.
Ionic liquid N,N,N',N'-tetramethylguanidinium trifluoroacetate (TMGT) has been applied as a green and reusable catalyst for the one-pot synthesis of 10-aryl substituted-9,10-dihydropyrano[2,3-h]chromene-2,8-diones via reaction of various aromatic aldehydes, 5,7-Dihydroxycoumarin derivatives and Meldrum's acid. The reactions were rapid, clean and the products were prepared in good yield. The ionic liquid was stable during the reaction process and reused without significant loss of its activity. The synthesised compounds were evaluated for their antioxidant activity by a 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay.
A new lignan with hypoglycemic activity from Tadehagi triquetrum.[Pubmed:25612052]
Nat Prod Res. 2015;29(18):1723-7.
A new lignan named tadehaginosin, together with a known compound 3,4-dihydro-4-(4'-hydroxyphenyl)-5,7-Dihydroxycoumarin, was isolated from the aerial part of Tadehagi triquetrum. The new structure was determined by various spectroscopic techniques ((1)H and (13)C APT, HSQC, HMBC, (1)H-(1)HCOSY, NOESY and HR-ESI-MS). The two isolates were evaluated for their hypoglycemic effects in vitro. Biological investigation showed that both of them possessed the capability to increase glucose consumption by HepG2 cells.
Chemistry and biological activity of coumarins at molecular level.[Pubmed:25233580]
Nat Prod Commun. 2014 Aug;9(8):1091-4.
Synthetic coumarins were prepared in high yields using ionic liquids as an environmental friendly alternative. 3,4-Dimethyl-7-hydroxycoumarin (3ab) and 3-isopropyl-4-methyl-5,7-Dihydroxycoumarin (3bc) showed interesting activity against Taq DNA polymerase with IC50 values of 115.7 microM and 82.2 microM, respectively. Also, 4-methyl-7-hydroxycoumarin (3aa) and 4-methyl-5,7-Dihydroxycoumarin (3ba) exhibited inhibitory activity against MMLV-RT with IC50 values of 23.5 microM and 18.3 microM, respectively. These inhibitors could have importance as antiretroviral chemotherapeutic agents and also for the development of antitumor drugs.
Evaluation of coumarin and neoflavone derivatives as HCV NS5B polymerase inhibitors.[Pubmed:23311976]
Chem Biol Drug Des. 2013 May;81(5):607-14.
Coumarins and coumestans represent an important family of compounds with diverse pharmacological properties. We recently identified coumestans as novel inhibitors of hepatitis C virus NS5B polymerase and predicted their binding in thumb pocket-1 (TP-1) of NS5B. As the coumarins are structurally related to coumestans by virtue of their common A- and B-rings, we postulated them to also exhibit similar binding interaction with NS5B and inhibit its polymerase function. We therefore investigated 24 coumarin and neoflavone derivatives as candidate NS5B inhibitors and identified 14 compounds inhibiting NS5B polymerase activity with IC50 values between 17 and 63 mum. Of these, the newly synthesized 6,8-diallyl-5,7-Dihydroxycoumarin (8a) was produced in three steps in high chemical yield from floroglucinol and found to be the most potent of this series, exhibiting activity similar to the reference coumestan LQB-34. The binding site of 8a was mapped to TP-1 of NS5B by counter screening against P495L NS5B mutant, employed as a screen for TP-1 site binders. NS5B-TP-1-8a interaction map provided insight into 8a binding and offered clues for future SAR optimization.
Flavonoids with antiplasmodial and cytotoxic activities of Macaranga triloba.[Pubmed:22561914]
Fitoterapia. 2012 Jul;83(5):968-72.
A new flavanone derivative, malaysianone A (1), four prenylated flavanones, 6-prenyl-3'-methoxyeriodictyol (2), nymphaeol B (3), nymphaeol C (4) and 6-farnesyl-3',4',5,7-tetrahydroxyflavanone (5), and two coumarins, 5,7-Dihydroxycoumarin (6) and scopoletin (7), were isolated from the dichloromethane extract of the inflorescences of Macaranga triloba. The structures of these compounds were elucidated based on spectroscopic methods including nuclear magnetic resonance (NMR-1D and 2D), UV, IR and mass spectrometry. The cytotoxic activity of the compounds was tested against several cell lines, with 5 inhibiting very strongly the growth of HeLa and HL-60 cells (IC(50): 1.3 mug/ml and 3.3 mug/ml, respectively). Compound 5 also showed strong antiplasmodial activity (IC(50): 0.06 muM).
Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells.[Pubmed:21772233]
Molecules. 2011 Jul 19;16(7):6010-22.
A new arylbenzofuran, 3',5'-dihydroxy-6-methoxy-7-prenyl-2-arylbenzofuran (1), and 25 known compounds, including moracin R (2), moracin C (3), moracin O (4), moracin P (5), artoindonesianin O (6), moracin D (7), alabafuran A (8), mulberrofuran L (9), mulberrofuran Y (10), kuwanon A (11), kuwanon C (12), kuwanon T (13), morusin (14), kuwanon E (15), sanggenon F (16), betulinic acid (17), uvaol (18), ursolic acid (19), beta-sitosterol (20), oxyresveratrol 2-O-beta-D-glucopyranoside (21), mulberroside A (22), mulberroside B (23), 5,7-Dihydroxycoumarin 7-O-beta-D-glucopyranoside (24), 5,7-Dihydroxycoumarin 7-O-beta-D-apiofuranosyl-(1-->6)-O-beta-D-glucopyranoside (25) and adenosine (26), were isolated from Morus alba var. multicaulis Perro. (Moraceae). Their structures were determined by spectroscopic methods. The prenyl-flavonoids 11-14, 16, triterpenoids 17,18 and 20 showed significant inhibitory activity towards the differentiation of 3T3-L1 adipocytes. The arylbenzofurans 1-10 and prenyl-flavonoids 11-16 also showed significant nitric oxide (NO) production inhibitory effects in RAW264.7 cells.
Synthesis and evaluation of antibacterial activities of 5,7-dihydroxycoumarin derivatives.[Pubmed:21433055]
Arch Pharm (Weinheim). 2011 Jun;344(6):386-93.
This study examines the synthesis and antibacterial activities of 5,7-Dihydroxycoumarin derivatives, whose structures were confirmed using analytical and spectral data. Twenty compounds were tested for their antibacterial activities against five microbial species such as E. coli, S. aureus, K. pneumonia, P. aeruginosa, and S. typhimurium were studied. Compounds 5 and 12 exhibited the most potent activity against Staphylococcus aureus with a MIC value of 2.5 microg/mL for each of the compounds.
A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins.[Pubmed:19155158]
Int J Antimicrob Agents. 2009 May;33(5):421-6.
The antimicrobial activities of 43 naturally occurring and synthetic coumarins were studied. Using a microtitre assay developed in-house, a range of Gram-positive and Gram-negative bacteria, including a hospital isolate of methicillin-resistant Staphylococcus aureus (MRSA),were utilised. The coumarins exhibiting good bioactivity (i.e. a low minimum inhibitory concentration) against two S. aureus strains were then assessed for their antimicrobial activities against a range of eight clinically isolated MRSA strains. The study showed that nearly one-half of the tested compounds displayed antimicrobial activity. Sixteen of these coumarins also possessed resistance-modifying activity, which reversed the resistance mechanism in MRSA allowing the antimicrobial oxacillin to exert an enhanced effect against an MRSA hospital strain. When tested in combination with oxacillin, 8-iodo-5,7-Dihydroxycoumarin (32) had a similar activity to vancomycin, which is the current drug of choice for the treatment of MRSA infections.
Neoflavonoids from Polygonum perfoliatum.[Pubmed:17260292]
Planta Med. 1999 Oct;65(7):671-3.
Four neoflavonoids, 3,4-dihydro-4-(4'-hydroxyphenyl)-5,7-Dihydroxycoumarin (1), 3,4-dihydro-5-hydroxy-7-methoxy-4-(4'-methoxyphenyl)coumarin (2), 3,4-dihydro-5-hydroxy-4-(4'-hydroxyphenyl)-7-methoxycoumarin (3), and 3,4-dihydro-5,7-dihydroxy-4-(4'-methoxyphenyl)coumarin (4), were isolated from the whole plant of Polygonum perfoliatum. Neoflavonoids 1, 2, and 4 were known compounds, but 3 is a new member of this class.


