DeoxyshikoninCAS# 43043-74-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 43043-74-9 | SDF | Download SDF |
| PubChem ID | 98914 | Appearance | Powder |
| Formula | C16H16O4 | M.Wt | 272.3 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
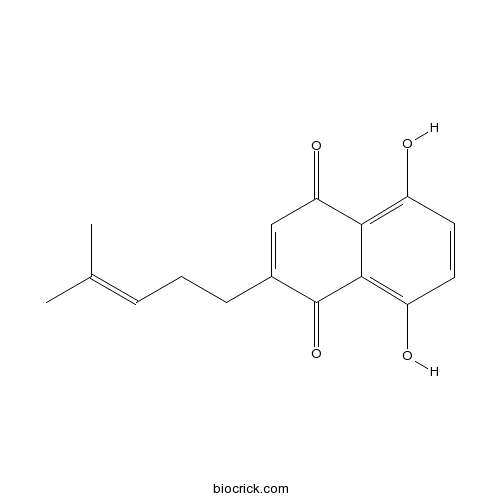
| Chemical Name | 5,8-dihydroxy-2-(4-methylpent-3-enyl)naphthalene-1,4-dione | ||
| SMILES | CC(=CCCC1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)C | ||
| Standard InChIKey | VOMDIEGPEURZJO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16O4/c1-9(2)4-3-5-10-8-13(19)14-11(17)6-7-12(18)15(14)16(10)20/h4,6-8,17-18H,3,5H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Deoxyshikonin and dodecyl gallate show significantly synergic antimicrobial activity with penicillin in vivo and in vitro, and can effectively reduce nasopharyngeal and lung colonization caused by different penicillin-resistant pneumococcal serotypes. Deoxyshikonin exerts very good radical scavenging activities toward ABTS+ but shows moderate inhibition of DPPH·, and shows cytotoxic activities. Deoxyshikonin may be a new drug candidate for wound healing and treatment of lymphatic diseases. |
| Targets | VEGFR | HIF | ERK | p38MAPK | Antifection |
| In vitro | Enhancement of Lymphangiogenesis In Vitro via the Regulations of HIF-1α Expression and Nuclear Translocation by Deoxyshikonin.[Pubmed: 23737816]Evid Based Complement Alternat Med. 2013;2013:148297.The objectives of this study were to determine the effects of Deoxyshikonin on lymphangiogenesis. Antibacterial effects of Traditional Chinese Medicine monomers against Streptococcus pneumoniae via inhibiting pneumococcal histidine kinase (VicK).[Pubmed: 26042111]Front Microbiol. 2015 May 20;6:479.Two-component systems (TCSs) have the potential to be an effective target of the antimicrobials, and thus received much attention in recent years. VicK/VicR is one of TCSs in Streptococcus pneumoniae (S. pneumoniae), which is essential for pneumococcal survival. We have previously obtained several Traditional Chinese Medicine monomers using a computer-based screening. Antioxidants from a Chinese medicinal herb - Lithospermum erythrorhizon[Reference: WebLink]Food Chemistry, 2008 , 106 (1) :2-10.Seven compounds, Deoxyshikonin (1), β,β-dimethylacrylshikonin (2), isobutylshikonin (3), shikonin (4), 5,8-dihydroxy-2-(1-methoxy-4-methyl-3-pentenyl)-1,4-naphthalenedione (5), β-sitosterol (6) and a mixture of two caffeic acid esters [7 (7a,7b)] were isolated from Lithospermum erythrorhizon Sieb et. Zucc. and identified by spectroscopic methods. Among them, compound 5 was isolated from this plant species for the first time. |
| Cell Research | Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem.[Pubmed: 28435429 ]Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies.[Pubmed: 27725239 ]J Ethnopharmacol. 2017 Mar 6;199:128-137.Jawoongo is a traditional drug ointment (with a traditional botanic formula) used for the treatment of burns and wounds in Korea. One of the components of Jawoongo is Lithospermi Radix (LR, the dried root of Lithospermum erythrorhizon Siebold & Zucc., also known as Zicao or Gromwell), which contains Deoxyshikonin and its derivatives.
The aim of the present study was to investigate the effects of Deoxyshikonin on wound healing.
EXCLI J. 2017 Feb 16;16:73-88.In this study, the antibacterial and cytotoxic activities of isolated compounds from the roots of Onosma visianii were investigated. |

Deoxyshikonin Dilution Calculator

Deoxyshikonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6724 mL | 18.3621 mL | 36.7242 mL | 73.4484 mL | 91.8105 mL |
| 5 mM | 0.7345 mL | 3.6724 mL | 7.3448 mL | 14.6897 mL | 18.3621 mL |
| 10 mM | 0.3672 mL | 1.8362 mL | 3.6724 mL | 7.3448 mL | 9.1811 mL |
| 50 mM | 0.0734 mL | 0.3672 mL | 0.7345 mL | 1.469 mL | 1.8362 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3672 mL | 0.7345 mL | 0.9181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vinpocetine
Catalog No.:BCN2609
CAS No.:42971-09-5
- Cathepsin G Inhibitor I
Catalog No.:BCC3598
CAS No.:429676-93-7
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- Nabumetone
Catalog No.:BCC4434
CAS No.:42924-53-8
- Z-Abu-OH
Catalog No.:BCC3201
CAS No.:42918-86-5
- Cladribine
Catalog No.:BCC1173
CAS No.:4291-63-8
- Tilianin
Catalog No.:BCN3669
CAS No.:4291-60-5
- Santamarine
Catalog No.:BCN5485
CAS No.:4290-13-5
- 14-Deoxy-11,12-didehydroandrographolide
Catalog No.:BCN1441
CAS No.:42895-58-9
- For-Met-OH
Catalog No.:BCC2992
CAS No.:4289-98-9
- 2-(3-Benzoylphenyl)propionitrile
Catalog No.:BCC8479
CAS No.:42872-30-0
- H-Ala-OBzl.TosOH
Catalog No.:BCC3191
CAS No.:42854-62-6
- Umckalin
Catalog No.:BCC9211
CAS No.:43053-62-9
- DPO-1
Catalog No.:BCC7398
CAS No.:43077-30-1
- Necrostatin-1
Catalog No.:BCC2247
CAS No.:4311-88-0
- 7'-O-Ethylmarmin
Catalog No.:BCC8274
CAS No.:
- SJ 172550
Catalog No.:BCC2416
CAS No.:431979-47-4
- Allylestrenol
Catalog No.:BCC8814
CAS No.:432-60-0
- Zopiclone
Catalog No.:BCC9195
CAS No.:43200-80-2
- 6-(5-Chloropyridin-2-yl)-7-hydroxy-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-one
Catalog No.:BCC8753
CAS No.:43200-81-3
- Skp2 Inhibitor C1
Catalog No.:BCC6298
CAS No.:432001-69-9
- Fenbendazole
Catalog No.:BCC1236
CAS No.:43210-67-9
- Formoterol Hemifumarate
Catalog No.:BCC4349
CAS No.:43229-80-7
- 6-Hydroxykaempferol
Catalog No.:BCN3334
CAS No.:4324-55-4
Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem.[Pubmed:28435429]
EXCLI J. 2017 Feb 16;16:73-88.
In this study, the antibacterial and cytotoxic activities of isolated compounds from the roots of Onosma visianii were investigated. By using different chromatographic techniques and appropriate spectroscopic methods, the seven naphthoquinones were described: Deoxyshikonin ( 1 ), isobutyrylshikonin ( 2 ), alpha-methylbutyrylshikonin ( 3 ), acetylshikonin ( 4 ), beta-hydroxyisovalerylshikonin ( 5 ), 5,8-O-dimethyl isobutyrylshikonin ( 6 ) and 5,8-O-dimethyl Deoxyshikonin ( 7 ). Among the tested compounds, 3 and 4 exhibited the highest antibacterial activities toward all tested bacterial species (MIC50 and MIC90 for gram positive bacteria: 6.40 mug/mL-12.79 mug/mL and 6.82 mug/mL-13.60 mug/mL, respectively; for gram negative bacteria: 4.27 mug/mL-8.53 mug/mL and 4.77 mug/mL-9.54 mug/mL, respectively). Also, naphthoquinones 3 and 4 exhibited strong cytotoxic activity against MDA-MB-231 cells (IC50 values 86.0 mug/mL and 80.2 mug/mL, respectively), while compounds 1 , 3 , 4 and 5 significantly decreased viability of HCT116 cells (IC50 values of 97.8 mug/mL, 15.2 mug/mL, 24.6 mug/mL and 30.9 mug/mL, respectively). Our results indicated that all tested naphthoquinone pigments are potential candidates for clinical uses as antibacterial and cytotoxic agents.
Enhancement of Lymphangiogenesis In Vitro via the Regulations of HIF-1alpha Expression and Nuclear Translocation by Deoxyshikonin.[Pubmed:23737816]
Evid Based Complement Alternat Med. 2013;2013:148297.
The objectives of this study were to determine the effects of Deoxyshikonin on lymphangiogenesis. Deoxyshikonin enhanced the ability of human dermal lymphatic microvascular endothelial cells (HMVEC-dLy) to undergo time-dependent in vitro cord formation. Interestingly, an opposite result was observed in cells treated with shikonin. The increased cord formation ability following Deoxyshikonin treatment correlated with increased VEGF-C mRNA expression to higher levels than seen for VEGF-A and VEGF-D mRNA expression. We also found that Deoxyshikonin regulated cord formation of HMVEC-dLy by increasing the HIF-1 alpha mRNA level, HIF-1 alpha protein level, and the accumulation of HIF-1 alpha in the nucleus. Knockdown of the HIF-1 alpha gene by transfection with siHIF-1 alpha decreased VEGF-C mRNA expression and cord formation ability in HMVEC-dLy. Deoxyshikonin treatment could not recover VEGF-C mRNA expression and cord formation ability in HIF-1 alpha knockdown cells. This indicated that Deoxyshikonin induction of VEGF-C mRNA expression and cord formation in HMVEC-dLy on Matrigel occurred mainly via HIF-1 alpha regulation. We also found that Deoxyshikonin promoted wound healing in vitro by the induction of HMVEC-dLy migration into the wound gap. This study describes a new effect of Deoxyshikonin, namely, the promotion of cord formation by human endothelial cells via the regulation of HIF-1 alpha . The findings suggest that Deoxyshikonin may be a new drug candidate for wound healing and treatment of lymphatic diseases.
Antibacterial effects of Traditional Chinese Medicine monomers against Streptococcus pneumoniae via inhibiting pneumococcal histidine kinase (VicK).[Pubmed:26042111]
Front Microbiol. 2015 May 20;6:479.
Two-component systems (TCSs) have the potential to be an effective target of the antimicrobials, and thus received much attention in recent years. VicK/VicR is one of TCSs in Streptococcus pneumoniae (S. pneumoniae), which is essential for pneumococcal survival. We have previously obtained several Traditional Chinese Medicine monomers using a computer-based screening. In this study, either alone or in combination with penicillin, their antimicrobial activities were evaluated based on in vivo and in vitro assays. The results showed that the MICs of 5'-(Methylthio)-5'-deoxyadenosine, octanal 2, 4-dinitrophenylhydrazone, Deoxyshikonin, kavahin, and dodecyl gallate against S. pneumoniae were 37.1, 38.5, 17, 68.5, and 21 mug/mL, respectively. Time-killing assays showed that these compounds elicited bactericidal effects against S. pneumoniae D39 strain, which led to a 6-log reduction in CFU after exposure to compounds at four times of the MIC for 24 h. The five compounds inhibited the growth of Streptococcus pyogenes, Streptococcus mitis, Streptococcus mutans or Streptococcus pseudopneumoniae, meanwhile, Deoxyshikonin and dodecyl gallate displayed strong inhibitory activities against Staphylococcus aureus. These compounds showed no obvious cytotoxicity effects on Vero cells. Survival time of the mice infected by S. pneumoniae strains was prolonged by the treatment with the compounds. Importantly, all of the five compounds exerted antimicrobial effects against multidrug-resistant clinical strains of S. pneumoniae. Moreover, even at sub-MIC concentration, they inhibited cell division and biofilm formation. The five compounds all have enhancement effect on penicillin. Deoxyshikonin and dodecyl gallate showed significantly synergic antimicrobial activity with penicillin in vivo and in vitro, and effectively reduced nasopharyngeal and lung colonization caused by different penicillin-resistant pneumococcal serotypes. In addition, the two compounds also showed synergic antimicrobial activity with erythromycin and tetracycline. Taken together, our results suggest that these novel VicK inhibitors may be promising compounds against the pneumococcus, including penicillin-resistant strains.


