Cathepsin G Inhibitor ICathepsin G inhibitor CAS# 429676-93-7 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
- SID 26681509
Catalog No.:BCC2362
CAS No.:958772-66-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 429676-93-7 | SDF | Download SDF |
| PubChem ID | 9830518 | Appearance | Powder |
| Formula | C36H33N2O6P | M.Wt | 620.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >10.4mg/mL in DMSO | ||
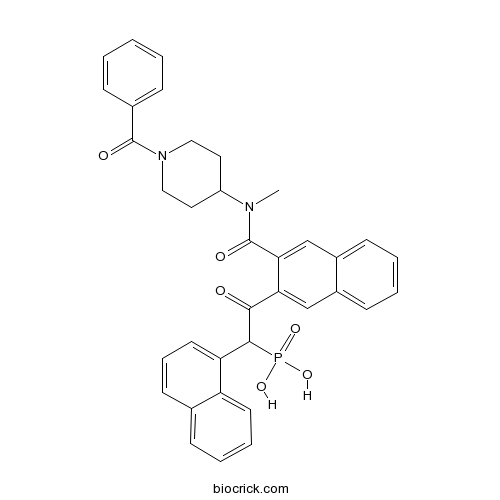
| Chemical Name | [2-[3-[(1-benzoylpiperidin-4-yl)-methylcarbamoyl]naphthalen-2-yl]-1-naphthalen-1-yl-2-oxoethyl]phosphonic acid | ||
| SMILES | CN(C1CCN(CC1)C(=O)C2=CC=CC=C2)C(=O)C3=CC4=CC=CC=C4C=C3C(=O)C(C5=CC=CC6=CC=CC=C65)P(=O)(O)O | ||
| Standard InChIKey | GNOZQRKYZJSIPZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C36H33N2O6P/c1-37(28-18-20-38(21-19-28)35(40)25-11-3-2-4-12-25)36(41)32-23-27-14-6-5-13-26(27)22-31(32)33(39)34(45(42,43)44)30-17-9-15-24-10-7-8-16-29(24)30/h2-17,22-23,28,34H,18-21H2,1H3,(H2,42,43,44) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cathepsin G Inhibitor I is a potent, selective, reversible, competitive, non-peptide inhibitor of cathepsin G. | |||||
| Targets | cathepsins G | |||||
| IC50 | 53nM | |||||

Cathepsin G Inhibitor I Dilution Calculator

Cathepsin G Inhibitor I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6112 mL | 8.0562 mL | 16.1124 mL | 32.2248 mL | 40.281 mL |
| 5 mM | 0.3222 mL | 1.6112 mL | 3.2225 mL | 6.445 mL | 8.0562 mL |
| 10 mM | 0.1611 mL | 0.8056 mL | 1.6112 mL | 3.2225 mL | 4.0281 mL |
| 50 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6445 mL | 0.8056 mL |
| 100 mM | 0.0161 mL | 0.0806 mL | 0.1611 mL | 0.3222 mL | 0.4028 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 53 nM
Cathepsin G (EC 3.4.21.20, chymotrypsin-like proteinase, neutral proteinase) is an enzymatic protein belonging to the peptidase or protease families. In humans, it is coded by the CTSG gene. Cathepsin G Inhibitor I is a potent, selective, reversible, competitive, non-peptide inhibitor of cathepsin G.
In vitro: Cathepsin G Inhibitor I shows reversible, competitive inhibition with IC50 and Ki values of 53 ± 12 (N = 10) and 63 ± 14 nM (N = 5), respectively. Another attribute of Cathepsin G Inhibitor I relates to its selectivity vs other serine proteases. It weakly inhibits chymotrypsin (Ki = 1.5 ± 0.2 μM), and poorly inhibits (<50% inhibition at 100 μM) thrombin, factor Xa, factor IXa, plasmin, trypsin, tryptase, proteinase 3, and human leukocyte elastase [1].
In vivo: Cathepsin G Inhibitor I is currently in in-vitro investigation and no animal in vivo study is ongoing [1].
Clinical trial: Cathepsin G Inhibitor I is currently in the preclinical development and no clinical trial is ongoing.
Reference:
[1] Greco MN, Hawkins MJ, Powell ET, Almond HR Jr, Corcoran TW, de Garavilla L, Kauffman JA, Recacha R, Chattopadhyay D, Andrade-Gordon P, Maryanoff BE. Nonpeptide inhibitors of cathepsin G: optimization of a novel beta-ketophosphonic acid lead by structure-based drug design. J Am Chem Soc. 2002;124(15):3810-1.
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- Nabumetone
Catalog No.:BCC4434
CAS No.:42924-53-8
- Z-Abu-OH
Catalog No.:BCC3201
CAS No.:42918-86-5
- Cladribine
Catalog No.:BCC1173
CAS No.:4291-63-8
- Tilianin
Catalog No.:BCN3669
CAS No.:4291-60-5
- Santamarine
Catalog No.:BCN5485
CAS No.:4290-13-5
- 14-Deoxy-11,12-didehydroandrographolide
Catalog No.:BCN1441
CAS No.:42895-58-9
- For-Met-OH
Catalog No.:BCC2992
CAS No.:4289-98-9
- 2-(3-Benzoylphenyl)propionitrile
Catalog No.:BCC8479
CAS No.:42872-30-0
- H-Ala-OBzl.TosOH
Catalog No.:BCC3191
CAS No.:42854-62-6
- Flumequine sodium
Catalog No.:BCC8985
CAS No.:42835-68-7
- Flumequine
Catalog No.:BCC5090
CAS No.:42835-25-6
- Vinpocetine
Catalog No.:BCN2609
CAS No.:42971-09-5
- Deoxyshikonin
Catalog No.:BCN3006
CAS No.:43043-74-9
- Umckalin
Catalog No.:BCC9211
CAS No.:43053-62-9
- DPO-1
Catalog No.:BCC7398
CAS No.:43077-30-1
- Necrostatin-1
Catalog No.:BCC2247
CAS No.:4311-88-0
- 7'-O-Ethylmarmin
Catalog No.:BCC8274
CAS No.:
- SJ 172550
Catalog No.:BCC2416
CAS No.:431979-47-4
- Allylestrenol
Catalog No.:BCC8814
CAS No.:432-60-0
- Zopiclone
Catalog No.:BCC9195
CAS No.:43200-80-2
- 6-(5-Chloropyridin-2-yl)-7-hydroxy-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-one
Catalog No.:BCC8753
CAS No.:43200-81-3
- Skp2 Inhibitor C1
Catalog No.:BCC6298
CAS No.:432001-69-9
- Fenbendazole
Catalog No.:BCC1236
CAS No.:43210-67-9
Using a Caesalpinia echinata Lam. protease inhibitor as a tool for studying the roles of neutrophil elastase, cathepsin G and proteinase 3 in pulmonary edema.[Pubmed:24140156]
Phytochemistry. 2013 Dec;96:235-43.
Acute lung injury (ALI) is characterized by neutrophil infiltration and the release of proteases, mainly elastase (NE), cathepsin G (Cat G) and proteinase 3 (PR3), which can be controlled by specific endogenous inhibitors. However, inhibitors of these proteases have been isolated from different sources, including plants. For this study, CeEI, or Caesalpinia echinata elastase inhibitor, was purified from C. echinata (Brazil-wood) seeds after acetone fractionation, followed by ion exchange and reversed phase chromatographic steps. Characterization with SDS-PAGE, stability assays, amino acid sequencing and alignment with other protein sequences confirmed that CeEI is a member of the soybean Kunitz trypsin inhibitor family. Like other members of this family, CeEI is a 20 kDa monomeric protein; it is stable within a large pH and temperature range, with four cysteine residues forming two disulfide bridges, conserved amino acid residues and leucine-isoleucine residues in the reactive site. CeEI was able to inhibit NE and Cat G at a nanomolar range (with K(i)s of 1.9 and 3.6 nM, respectively) and inhibited PR3 within a micromolar range (K(i) 3.7 muM), leading to hydrolysis of specific synthetic substrates. In a lung edema model, CeEI reduced the lung weight and pulmonary artery pressure until 180 min after the injection of zymosan-activated polymorphonuclear neutrophils. In experiments performed in the presence of a Cat G and PR3, but not an NE inhibitor, lung edema was reduced only until 150 min and pulmonary artery pressure was similar to that of the control. These results confirm that NE action is crucial to edema establishment and progression. Additionally, CeEI appears to be a useful tool for studying the physiology of pulmonary edema and provides a template for molecular engineering and drug design for ALI therapy.
Design of Potent and Selective Cathepsin G Inhibitors Based on the Sunflower Trypsin Inhibitor-1 Scaffold.[Pubmed:28045523]
J Med Chem. 2017 Jan 26;60(2):658-667.
Neutrophils are directly responsible for destroying invading pathogens via reactive oxygen species, antimicrobial peptides, and neutrophil serine proteases (NSPs). Imbalance between NSP activity and endogenous protease inhibitors is associated with chronic inflammatory disorders, and engineered inhibitors of NSPs are a potential therapeutic pathway. In this study we characterized the extended substrate specificity (P4-P1) of the NSP cathepsin G using a peptide substrate library. Substituting preferred cathepsin G substrate sequences into sunflower trypsin inhibitor-1 (SFTI-1) produced a potent cathepsin G inhibitor (Ki = 0.89 nM). Cathepsin G's P2' preference was determined by screening against a P2' diverse SFTI-based library, and the most preferred residue at P2' was combined in SFTI-1 with a preferred substrate sequence (P4-P2) and a nonproteinogenic P1 residue (4-guanidyl-l-phenylalanine) to produce a potent (Ki = 1.6 nM) and the most selective (>/=360-fold) engineered cathepsin G inhibitor reported to date. This compound is a promising lead for further development of cathepsin G inhibitors targeting chronic inflammatory disorders.
DNA structures decorated with cathepsin G/secretory leukocyte proteinase inhibitor stimulate IFNI production by plasmacytoid dendritic cells.[Pubmed:23885335]
Am J Clin Exp Immunol. 2013 Jun 15;2(2):186-94. Print 2013.
Plasmacytoid dendritic cells (pDCs) and neutrophils are detected in psoriatic skin lesions and implicated in the pathogenesis of psoriasis. pDCs specialize in the production of type I interferon (IFNI), a cytokine that plays an important role in chronic autoimmune-like inflammation, including psoriasis. Here, we demonstrate that IFNI production in pDCs is stimulated by DNA structures containing the neutrophil serine protease cathepsin G (CatG) and the secretory leukocyte protease inhibitor (SLPI), which is a controlling inhibitor of serine proteases. We also demonstrate the presence of neutrophil-derived DNA structures containing CatG and SLPI in lesional skin samples from psoriasis patients. These findings suggest a previously unappreciated role for CatG in psoriasis by linking CatG and its inhibitor SLPI to the IFNI-dependent regulation of immune responses by pDCs in psoriatic skin.
Determination of cathepsin G in endometrial tissue using a surface plasmon resonance imaging biosensor with tailored phosphonic inhibitor.[Pubmed:25218550]
Eur J Obstet Gynecol Reprod Biol. 2014 Nov;182:38-42.
OBJECTIVE: Cathepsin G is a serine peptidase whose physiological role is mainly associated with an early immune response, anti-microbial activity as well as platelet activation or hydrolysis of coagulation factors. In addition, since the activity of cathepsin G has been associated with the development of various pathological disorders, the measurement of its activity in patient samples is of high interest. Unfortunately, the usefulness of common immunological methods is limited, since they cannot distinguish between catalytically active and inactive protease. STUDY DESIGN: Here we present the application of recently developed Surface Plasmon Resonance-based biosensor for the detection of active cathepsin G in human endometrium samples. The key element of the system is based on the irreversible binding of cathepsin G to its specific phosphonic-type inhibitor immobilized on the surface of the gold chip. The concentration of cathepsin G was measured in tissue samples from the group of patients with endometriosis as well as in the control group. RESULTS: The level of cathepsin G ascertained in endometrium tissue samples was over twice as high for the group of patients suffering from endometriosis as compared to the control group, with the median values of 0.5 pmol/mg and 0.2 pmol/mg, respectively. CONCLUSION: The SPR sensor armed with a specific irreversible phosphonic inhibitor represents a highly useful tool for the determination of catalytically active cathepsin G concentration in endometrial tissue.


