Formoterol HemifumarateCAS# 43229-80-7 |
- LY2801653 dihydrochloride
Catalog No.:BCC1721
CAS No.:1206801-37-7
- c-Met inhibitor 1
Catalog No.:BCC1488
CAS No.:1357072-61-7
- Sunitinib malate
Catalog No.:BCC3664
CAS No.:341031-54-7
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

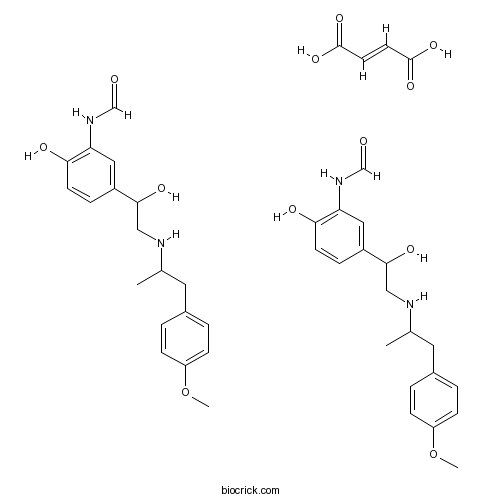
| Cas No. | 43229-80-7 | SDF | Download SDF |
| PubChem ID | 5282416 | Appearance | Powder |
| Formula | C42H52N4O12 | M.Wt | 804.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BD 40A | ||
| Solubility | DMSO : ≥ 50 mg/mL (124.24 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (E)-but-2-enedioic acid;N-[2-hydroxy-5-[1-hydroxy-2-[1-(4-methoxyphenyl)propan-2-ylamino]ethyl]phenyl]formamide | ||
| SMILES | CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O.CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | OBRNDARFFFHCGE-WXXKFALUSA-N | ||
| Standard InChI | InChI=1S/2C19H24N2O4.C4H4O4/c2*1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22;5-3(6)1-2-4(7)8/h2*3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22);1-2H,(H,5,6)(H,7,8)/b;;2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and long-acting β2-adrenoceptor agonist. Displays 330-fold selectivity for β2 over β1 receptors (pKd values are 8.12 and 5.58 respectively). Potently relaxes guinea pig trachea (pD2 = 9.29), and is longer-acting and 100-fold more potent than salbutamol. |

Formoterol Hemifumarate Dilution Calculator

Formoterol Hemifumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2424 mL | 6.2121 mL | 12.4242 mL | 24.8484 mL | 31.0605 mL |
| 5 mM | 0.2485 mL | 1.2424 mL | 2.4848 mL | 4.9697 mL | 6.2121 mL |
| 10 mM | 0.1242 mL | 0.6212 mL | 1.2424 mL | 2.4848 mL | 3.1061 mL |
| 50 mM | 0.0248 mL | 0.1242 mL | 0.2485 mL | 0.497 mL | 0.6212 mL |
| 100 mM | 0.0124 mL | 0.0621 mL | 0.1242 mL | 0.2485 mL | 0.3106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50 Value: 2.1nM in pregnant C3H/HeN strain mice[5] Formoterol is a potent, selective and long-acting β2-adrenoceptor agonist to β2 receptors.
- Fenbendazole
Catalog No.:BCC1236
CAS No.:43210-67-9
- Skp2 Inhibitor C1
Catalog No.:BCC6298
CAS No.:432001-69-9
- 6-(5-Chloropyridin-2-yl)-7-hydroxy-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-one
Catalog No.:BCC8753
CAS No.:43200-81-3
- Zopiclone
Catalog No.:BCC9195
CAS No.:43200-80-2
- Allylestrenol
Catalog No.:BCC8814
CAS No.:432-60-0
- SJ 172550
Catalog No.:BCC2416
CAS No.:431979-47-4
- 7'-O-Ethylmarmin
Catalog No.:BCC8274
CAS No.:
- Necrostatin-1
Catalog No.:BCC2247
CAS No.:4311-88-0
- DPO-1
Catalog No.:BCC7398
CAS No.:43077-30-1
- Umckalin
Catalog No.:BCC9211
CAS No.:43053-62-9
- Deoxyshikonin
Catalog No.:BCN3006
CAS No.:43043-74-9
- Vinpocetine
Catalog No.:BCN2609
CAS No.:42971-09-5
- 6-Hydroxykaempferol
Catalog No.:BCN3334
CAS No.:4324-55-4
- Boc-Tyr-OMe
Catalog No.:BCC3459
CAS No.:4326-36-7
- Toddaculine
Catalog No.:BCN3639
CAS No.:4335-12-0
- 3-O-Acetyloleanolic acid
Catalog No.:BCN5486
CAS No.:4339-72-4
- VU 0357121
Catalog No.:BCC4595
CAS No.:433967-28-3
- Ethisterone
Catalog No.:BCC4478
CAS No.:434-03-7
- Methenolone acetate
Catalog No.:BCC9028
CAS No.:434-05-9
- Oxymetholone
Catalog No.:BCC4692
CAS No.:434-07-1
- Lithocholic Acid
Catalog No.:BCC3805
CAS No.:434-13-9
- Nandrolone
Catalog No.:BCC9086
CAS No.:434-22-0
- Dacarbazine
Catalog No.:BCC1174
CAS No.:4342-03-4
- K 41498
Catalog No.:BCC5867
CAS No.:434938-41-7
The role of beta-adrenergic receptors in the cardioprotective effects of beta-preconditioning (betaPC).[Pubmed:21225332]
Cardiovasc Drugs Ther. 2011 Feb;25(1):31-46.
AIM: To determine the mechanism whereby transient stimulation of the beta-adrenergic receptor subtypes (beta-AR) elicit cardioprotection against subsequent ischaemia. METHODS: Isolated rat hearts were subjected to 35 min regional ischaemia (RI) and reperfusion and infarct size (IS) determined. Hearts were preconditioned with 5 min isoproterenol (beta1/beta2-AR agonist), denopamine (beta1-AR agonist), Formoterol Hemifumarate (beta2-AR agonist) or BRL37344 (beta3-AR agonist) and 5 min reperfusion. The roles of the beta-ARs, NO, PKA, and PI3-K were explored by using selective antagonists/blockers. Pertussis toxin was administered i.p., 48 h prior to experimentation. RESULTS: IS of hearts preconditioned with either isoproterenol, denopamine or formoterol (% of area at risk: 23.6 +/- 1.26; 24.52 +/- 0.89; 20.74 +/- 0.85 respectively) were significantly smaller than that of non-preconditioned hearts (41.7 +/- 1.65) and associated with improvement in postischaemic mechanical performance. The beta3-AR agonist BRL37344 could not reduce IS. The beta1- and beta2-AR blockers CGP-20712A and ICI-118551 abolished the reduction in IS and improvement in mechanical recovery during reperfusion induced by isoproterenol preconditioning, while the beta3-AR blocker SR59230A was without effect. Both Rp-8-CPT-cAMPs and wortmannin significantly increased IS when administered before and during beta1/beta2-AR preconditioning and reduced mechanical recovery. PTX pretreatment had no significant effect on the reduction in IS induced by beta1/beta2-AR or beta2-AR preconditioning, but reduced mechanical recovery in beta2-AR preconditioning. Similarly the NOS inhibitors L-NAME and LNNA had no effect on IS in beta1/beta2-AR preconditioning, but depressed mechanical recovery. CONCLUSION: Protection afforded by beta-ARs stimulation, depends on activation of both beta1-AR and beta2-ARs but not beta3-AR. With functional recovery as endpoint, results suggest involvement of NO in beta1/beta2-AR preconditioning and the Gi protein in beta2-AR preconditioning. Both PKA and PI3-K activation were essential for beta1/beta2-AR cardioprotection.
Expression of inwardly rectifying potassium channels (GIRKs) and beta-adrenergic regulation of breast cancer cell lines.[Pubmed:15603589]
BMC Cancer. 2004 Dec 16;4:93.
BACKGROUND: Previous research has indicated that at various organ sites there is a subset of adenocarcinomas that is regulated by beta-adrenergic and arachidonic acid-mediated signal transduction pathways. We wished to determine if this regulation exists in breast adenocarcinomas. Expression of mRNA that encodes a G-protein coupled inwardly rectifying potassium channel (GIRK1) has been shown in tissue samples from approximately 40% of primary human breast cancers. Previously, GIRK channels have been associated with beta-adrenergic signaling. METHODS: Breast cancer cell lines were screened for GIRK channels by RT-PCR. Cell cultures of breast cancer cells were treated with beta-adrenergic agonists and antagonists, and changes in gene expression were determined by both relative competitive and real time PCR. Potassium flux was determined by flow cytometry and cell signaling was determined by western blotting. RESULTS: Breast cancer cell lines MCF-7, MDA-MB-361 MDA-MB 453, and ZR-75-1 expressed mRNA for the GIRK1 channel, while MDA-MB-468 and MDA-MB-435S did not. GIRK4 was expressed in all six breast cancer cell lines, and GIRK2 was expressed in all but ZR-75-1 and MDA-MB-435. Exposure of MDA-MB-453 cells for 6 days to the beta-blocker propranolol (1 microM) increased the GIRK1 mRNA levels and decreased beta2-adrenergic mRNA levels, while treatment for 30 minutes daily for 7 days had no effect. Exposure to a beta-adrenergic agonist and antagonist for 24 hours had no effect on gene expression. The beta adrenergic agonist, Formoterol Hemifumarate, led to increases in K+ flux into MDA-MB-453 cells, and this increase was inhibited by the GIRK channel inhibitor clozapine. The tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a high affinity agonist for beta-adrenergic receptors stimulated activation of Erk 1/2 in MDA-MB-453 cells. CONCLUSIONS: Our data suggests beta-adrenergic receptors and GIRK channels may play a role in breast cancer.
Relaxant effects and durations of action of formoterol and salmeterol on the isolated human bronchus.[Pubmed:7914176]
Eur Respir J. 1994 May;7(5):914-20.
The objective of this study was to evaluate the potency and efficacy (intrinsic activity) of formoterol and salmeterol and their duration of action in comparison with other beta-adrenoceptor agonists in isolated human bronchi. Human bronchi were obtained at thoracotomy from patients with lung cancer. Potency (-log of the concentration of drug inducing 50% of maximal relaxation (-log EC50)) and efficacy (maximal effect (Emax), % of response to theophylline 3 x 10(-3) mol.l-1) were determined by analysis of cumulative isometric concentration-response curves to beta 2-adrenoceptor agonists in bronchial rings at resting tone or contracted maximally with acetylcholine 10(-3) mol.l-1 to induce functional antagonism. The onset and duration of action of beta-adrenoceptor agonists were measured by assessing the relaxant activity of drugs on the basal tone of isolated bronchi. In terms of potency, the rank order of the substances studied was formoterol > fenoterol > or = salmeterol > or = isoprenaline > or = salbutamol > or = adrenaline > or = terbutaline. Formoterol was 150-200 times more potent than isoprenaline. On preparations contracted with acetylcholine 10(-3) mol.l-1 the intrinsic activity (IA) of salbutamol, terbutaline and salmeterol compared with that of isoprenaline ranged 0.62-0.66. Intrinsic activity was higher with formoterol (0.84) and fenoterol (0.75). The onset of action of formoterol (2.14 +/- 0.55 min, n = 11) was not significantly different from that of salbutamol (1.90 +/- 0.24 min, n = 8) but shorter than that of salmeterol (6.40 +/- 1.40 min, n = 10).(ABSTRACT TRUNCATED AT 250 WORDS)
Formoterol: pharmacology, molecular basis of agonism, and mechanism of long duration of a highly potent and selective beta 2-adrenoceptor agonist bronchodilator.[Pubmed:8099696]
Life Sci. 1993;52(26):2145-60.
Formoterol is an innovative, highly potent, beta 2-adrenoceptor-selective agonist combining the clinical advantages of rapid onset of action with a duration of action in excess of 12 h. In vitro, formoterol is a potent airway smooth muscle relaxant with high efficacy, and very high affinity and selectivity for the beta 2-adrenoceptor. Formoterol appears to be retained in airway smooth muscle for extended periods since its relaxant effect on human airway smooth muscle is resistant to repeated washing and formoterol displays 'reassertion' of relaxation after washout of a beta-adrenoceptor antagonist. A model based on the diffusion microkinetics of formoterol into the plasmalemma lipid bilayer is proposed as a basis for these properties. In addition to the release of pro-inflammatory mediators from cells such as the mast cell, several other disease processes probably occur in asthma. Leukocytes, notably eosinophils, adhere to the vascular endothelium and emigrate into airway tissues, which may be damaged by these cells if they are activated to release mediators or their granular contents. Plasma and its component proteins are extravasated from the bronchial microcirculation. Formoterol has been demonstrated to potently inhibit these cells and processes in experimental test systems. Continuing clinical research involving histological examination of tissue reactions may allow a more complete determination of the effects of formoterol on inflammatory processes in humans and the clinical relevance of any such effects.
Effects of N-aralkyl substitution of beta-agonists on alpha- and beta-adrenoceptor subtypes: pharmacological studies and binding assays.[Pubmed:6121868]
J Pharm Pharmacol. 1982 Feb;34(2):107-12.
The pharmacological and binding properties of four beta-adrenomimetic drugs with N-alkyl substitutions (isoprenaline, terbutaline, salbutamol and soterenol) were compared with those of four corresponding drugs with N-aralkyl substitutions (protokylol, ME 506, salmefamol and zinterol). BD-40 A, a very powerful beta 2-agonist with a related chemical structure, was also included in this study. The beta 1- and beta 2-activities of these drugs were determined on guinea-pig atria and trachea, their alpha-adrenolytic activity was measured on rat aorta and their affinities (Ki) for alpha 1- and alpha 2-adrenoceptors on rat cortical membranes were assessed using [3H]prazosin and [3H]yohimbine. In this group of beta-agonists, substitution of the N-alkyl by an N-aralkyl group had a variable effect on the beta 2-selectivity whereas alpha-adrenolytic properties were always enhanced. An increase of the affinities (Ki) for both alpha 1- and alpha 2-adrenoceptors was found but the effect was much more pronounced for alpha 1-adrenoceptors. These results indicated that the alpha-adrenolytic activity observed with the N-aralkyl beta-agonists was selective for alpha 1-adrenoceptors.


