DiMNFAHR modulator CAS# 14756-24-2 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14756-24-2 | SDF | Download SDF |
| PubChem ID | 276138 | Appearance | Powder |
| Formula | C21H16O4 | M.Wt | 332.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in DMSO | ||
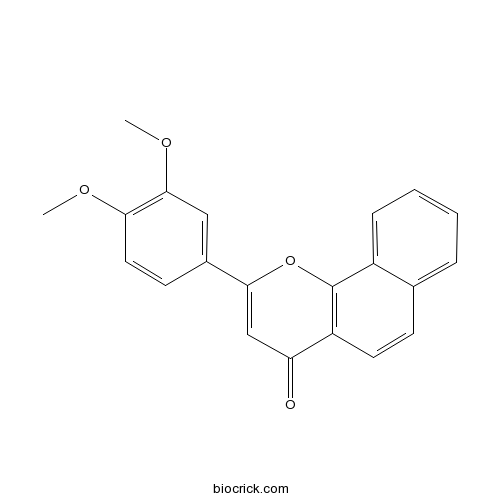
| Chemical Name | 2-(3,4-dimethoxyphenyl)benzo[h]chromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(O2)C4=CC=CC=C4C=C3)OC | ||
| Standard InChIKey | QDZQDIUUJDAORK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16O4/c1-23-18-10-8-14(11-20(18)24-2)19-12-17(22)16-9-7-13-5-3-4-6-15(13)21(16)25-19/h3-12H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective aryl hydrocarbon receptor (AHR) modulator (SAhRM). Suppresses expression of CD55 and CD46 induced by IL-1β in an inflammatory tumor cell microenvironment; exhibits no effect on basal CD55 or CD46 expression. |

DiMNF Dilution Calculator

DiMNF Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0089 mL | 15.0444 mL | 30.0888 mL | 60.1775 mL | 75.2219 mL |
| 5 mM | 0.6018 mL | 3.0089 mL | 6.0178 mL | 12.0355 mL | 15.0444 mL |
| 10 mM | 0.3009 mL | 1.5044 mL | 3.0089 mL | 6.0178 mL | 7.5222 mL |
| 50 mM | 0.0602 mL | 0.3009 mL | 0.6018 mL | 1.2036 mL | 1.5044 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3009 mL | 0.6018 mL | 0.7522 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective aryl hydrocarbon receptor (AHR) modulator (SAhRM). Suppresses expression of CD55 and CD46 induced by IL-1β in an inflammatory tumor cell microenvironment; exhibits no effect on basal CD55 or CD46 expression.
- Racemodine
Catalog No.:BCN2023
CAS No.:147554-28-7
- trans-3-Hydroxycinnamic acid
Catalog No.:BCN5029
CAS No.:14755-02-3
- Bosentan
Catalog No.:BCC4640
CAS No.:147536-97-8
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- LY 288513
Catalog No.:BCC5772
CAS No.:147523-65-7
- Thunberginol C
Catalog No.:BCN1654
CAS No.:147517-06-4
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
- Ginsenoside Rg6
Catalog No.:BCN2706
CAS No.:147419-93-0
- Telenzepine dihydrochloride
Catalog No.:BCC6946
CAS No.:147416-96-4
- Omaveloxolone (RTA-408)
Catalog No.:BCC5281
CAS No.:1474034-05-3
- Azilsartan
Catalog No.:BCC5014
CAS No.:147403-03-0
- Ligupurpuroside B
Catalog No.:BCC8199
CAS No.:147396-02-9
- ID-8
Catalog No.:BCC4787
CAS No.:147591-46-6
- Novobiocin Sodium
Catalog No.:BCC4812
CAS No.:1476-53-5
- 5-Chloro-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile
Catalog No.:BCC8744
CAS No.:147619-40-7
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Magnolianin
Catalog No.:BCN3985
CAS No.:147663-91-0
- ZM 226600
Catalog No.:BCC6831
CAS No.:147695-92-9
- 2-Amino-4-methylbenzothiazole
Catalog No.:BCC8533
CAS No.:1477-42-5
- WIN 18446
Catalog No.:BCC6273
CAS No.:1477-57-2
- 8-(3-Chlorostyryl)caffeine
Catalog No.:BCC7640
CAS No.:147700-11-6
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
Selective aryl hydrocarbon receptor modulator-mediated repression of CD55 expression induced by cytokine exposure.[Pubmed:22553215]
J Pharmacol Exp Ther. 2012 Aug;342(2):345-55.
Modulation of aryl hydrocarbon receptor (AHR) activity by a class of ligands termed selective AHR modulators (SAhRMs) has been demonstrated to attenuate proinflammatory gene expression and signaling, including repression of cytokine-mediated induction of acute-phase genes (e.g., Saa1). These effects are observed to occur through an AHR-dependent mechanism that does not require canonical signaling through dioxin response elements. Previously, we have demonstrated that the SAhRM 3',4'-dimethoxy-alpha-naphthoflavone (DiMNF) can repress the cytokine-mediated induction of complement factor genes. Here, we report that the activation of the AHR with DiMNF can suppress cytokine-mediated induction of the membrane complement regulatory protein CD55. When CD55 is expressed on host cells, it facilitates the decay of the complement component 3 (C3) convertase, thereby protecting the cell from complement-mediated lysis. Tumor cells often exhibit elevated CD55 expression on the cell surface in the inflammatory microenvironment of the tumor, and such enhanced expression could represent a means of escaping immune surveillance. DiMNF can repress the cytokine-mediated induction of CD55 mRNA and protein. Luciferase reporter analysis has identified possible response elements on the CD55 promoter, which may be targets for this repression. A C3 deposition assay with [(125)I]C3 revealed that repression of cytokine-mediated CD55 expression by DiMNF led to an increase of C3 deposition on the surface of Huh7 cells, which would likely stimulate the formation of the membrane attack complex. These results suggest that SAhRMs such as DiMNF have therapeutic potential in regulating the immune response to tumor formation.
Distinct roles for aryl hydrocarbon receptor nuclear translocator and ah receptor in estrogen-mediated signaling in human cancer cell lines.[Pubmed:22235307]
PLoS One. 2012;7(1):e29545.
The activated AHR/ARNT complex (AHRC) regulates the expression of target genes upon exposure to environmental contaminants such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Importantly, evidence has shown that TCDD represses estrogen receptor (ER) target gene activation through the AHRC. Our data indicates that AHR and ARNT act independently from each other at non-dioxin response element sites. Therefore, we sought to determine the specific functions of AHR and ARNT in estrogen-dependent signaling in human MCF7 breast cancer and human ECC-1 endometrial carcinoma cells. Knockdown of AHR with siRNA abrogates dioxin-inducible repression of estrogen-dependent gene transcription. Intriguingly, knockdown of ARNT does not effect TCDD-mediated repression of estrogen-regulated transcription, suggesting that AHR represses ER function independently of ARNT. This theory is supported by the ability of the selective AHR modulator 3',4'-dimethoxy-alpha-naphthoflavone (DiMNF) to repress estrogen-inducible transcription. Furthermore, basal and estrogen-activated transcription of the genes encoding cathepsin-D and pS2 are down-regulated in MCF7 cells but up-regulated in ECC-1 cells in response to loss of ARNT. These responses are mirrored at the protein level with cathepsin-D. Furthermore, knock-down of ARNT led to opposite but corresponding changes in estrogen-stimulated proliferation in both MCF7 and ECC-1 cells. We have obtained experimental evidence demonstrating a dioxin-dependent repressor function for AHR and a dioxin-independent co-activator/co-repressor function for ARNT in estrogen signalling. These results provide us with further insight into the mechanisms of transcription factor crosstalk and putative therapeutic targets in estrogen-positive cancers.
Suppression of cytokine-mediated complement factor gene expression through selective activation of the Ah receptor with 3',4'-dimethoxy-alpha-naphthoflavone.[Pubmed:21127131]
Mol Pharmacol. 2011 Mar;79(3):508-19.
We have characterized previously a class of aryl hydrocarbon receptor (AHR) ligand termed selective AHR modulators (SAhRMs). SAhRMs exhibit anti-inflammatory properties, including suppression of cytokine-mediated acute phase genes (e.g., Saa1), through dissociation of non-dioxin-response element (DRE) AHR activity from DRE-dependent xenobiotic gene expression. The partial AHR agonist alpha-naphthoflavone (alphaNF) mediates the suppressive, non-DRE dependent effects on SAA1 expression and partial DRE-mediated CYP1A1 induction. These observations suggest that alphaNF may be structurally modified to a derivative exhibiting only SAhRM activity. A screen of alphaNF derivatives identifies 3',4'-dimethoxy-alphaNF (DiMNF) as a candidate SAhRM. Competitive ligand binding validates DiMNF as an AHR ligand, and DRE-dependent reporter assays with quantitative mRNA analysis of AHR target genes reveal minimal agonist activity associated with AHR binding. Consistent with loss of agonist activity, DiMNF fails to promote AHR binding to DRE probes as determined through electromobility shift assay. Importantly, mRNA analysis indicates that DiMNF retains the suppressive capacity of alphaNF regarding cytokine-mediated SAA1 expression in Huh7 cells. Interestingly, predictive docking modeling suggests that DiMNF adopts a unique orientation within the AHR ligand binding pocket relative to alphaNF and may facilitate the rational design of additional SAhRMs. Microarray studies with a non-DRE binding but otherwise functional AHR mutant identified complement factor C3 as a potential SAhRM target. We confirmed this observation in Huh7 cells using 10 muM DiMNF, which significantly repressed C3 mRNA and protein. These data expand the classes of AHR ligands exerting DRE-independent anti-inflammatory SAhRM activity, suggesting SAhRMs may have application in the amelioration of inflammatory disorders.


