Docosyl ferulateCAS# 101927-24-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

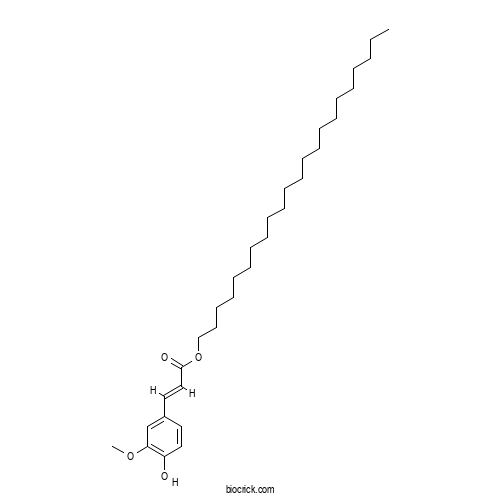
| Cas No. | 101927-24-6 | SDF | Download SDF |
| PubChem ID | 14238616.0 | Appearance | Powder |
| Formula | C32H54O4 | M.Wt | 502.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | docosyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CCCCCCCCCCCCCCCCCCCCCCOC(=O)C=CC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | USNYNNITUQSEEV-SHHOIMCASA-N | ||
| Standard InChI | InChI=1S/C32H54O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-27-36-32(34)26-24-29-23-25-30(33)31(28-29)35-2/h23-26,28,33H,3-22,27H2,1-2H3/b26-24+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Docosyl ferulate Dilution Calculator

Docosyl ferulate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9889 mL | 9.9447 mL | 19.8894 mL | 39.7788 mL | 49.7235 mL |
| 5 mM | 0.3978 mL | 1.9889 mL | 3.9779 mL | 7.9558 mL | 9.9447 mL |
| 10 mM | 0.1989 mL | 0.9945 mL | 1.9889 mL | 3.9779 mL | 4.9724 mL |
| 50 mM | 0.0398 mL | 0.1989 mL | 0.3978 mL | 0.7956 mL | 0.9945 mL |
| 100 mM | 0.0199 mL | 0.0994 mL | 0.1989 mL | 0.3978 mL | 0.4972 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neopuerarin A
Catalog No.:BCX1017
CAS No.:1150314-34-3
- Zeatin
Catalog No.:BCX1016
CAS No.:13114-27-7
- 6,8-Dihydroxy-1,2,7-trimethoxy-3-methylanthraquinone
Catalog No.:BCX1015
CAS No.:1622982-59-5
- Cycloastragenol-6-O-β-D-glucoside
Catalog No.:BCX1014
CAS No.:86764-12-7
- 6'-Hydroxy-3,4,2',3',4'-pentamethoxychalcone
Catalog No.:BCX1013
CAS No.:114021-62-4
- Isomaltotetraose
Catalog No.:BCX1012
CAS No.:35997-20-7
- Isomaltopentaose
Catalog No.:BCX1011
CAS No.:6082-32-2
- Isomaltohexaose
Catalog No.:BCX1010
CAS No.:6175-02-6
- Isomaltoheptaose
Catalog No.:BCX1009
CAS No.:6513-12-8
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Euphorbetin
Catalog No.:BCX1006
CAS No.:35897-99-5
- Furopelargone B
Catalog No.:BCX1019
CAS No.:1143-46-0
- Pseudostellarin A
Catalog No.:BCX1020
CAS No.:156430-20-5
- Soyasaponin Be
Catalog No.:BCX1021
CAS No.:117210-14-7
- Diphylloside A
Catalog No.:BCX1022
CAS No.:113558-11-5
- Pseudoginsenoside
Catalog No.:BCX1023
CAS No.:96158-07-5
- Notoginsenoside D
Catalog No.:BCX1024
CAS No.:193895-50-0
- Flavanone hydrazone
Catalog No.:BCX1025
CAS No.:1692-46-2
- Gallic acid 4-O-β-D-glucopyranoside
Catalog No.:BCX1026
CAS No.:84274-52-2
- 18α,20β-Glycyrrhizic acid
Catalog No.:BCX1027
CAS No.:83896-44-0
- 6-Hydroxymusizin 8-glucoside
Catalog No.:BCX1028
CAS No.:23566-96-3
- Ciwujianoside D2
Catalog No.:BCX1029
CAS No.:114892-57-8
- Ciwujianoside D1
Catalog No.:BCX1030
CAS No.:114912-35-5
The effect of isolates from Cassipourea flanaganii (Schinz) alston, a plant used as a skin lightning agent, on melanin production and tyrosinase inhibition.[Pubmed:32810622]
J Ethnopharmacol. 2021 Jan 10;264:113272.
ETHNOPHARMACOLOGICAL RELEVANCE: The Zulu and Xhosa people of South Africa use the stem bark of Cassipourea flanaganii as a skin-lightning cosmetic. AIM OF THE STUDY: To isolate and identify compounds responsible for the skin lightning properties from the stem bark of Cassipourea flanaganii and to evaluate their cytotoxicity towards skin cells. MATERIALS AND METHODS: Extracts from the stem bark of Cassipourea flanaganii were isolated using chromatographic methods and structures were determined using NMR, IR and MS analysis. The tyrosinase inhibitory activity and the ability to inhibit the production of melanin were determined using human primary epidermal melanocyte cells. Cytoxicity was established using the same melanocytes and a neutral red assay. RESULTS: One previously undescribed compound, ent-atis-16-en-19-al (1) along with the known ent-atis-16-en-19-oic acid (2), ent-atis-16-en-19-ol (3), ent-kaur-16-en-19-oic acid (4), ent-kaur-16-en-19-al (5), ent-manoyl oxide (6), guinesine A (7), guinesine B (8), guinesine C (9), lichenxanthone (10), 2,4-dihydroxy-3,6-dimethyl benzoic acid methyl ester (11), lynoside (12), lupeol (13), beta-amyrin (14), Docosyl ferulate (15), stigmasterol, sitosterol and sitosterol-O-glucoside were isolated in this investigation. An impure fraction containing compound 3 was acetylated to obtain 19-acetoxy-ent-atis-16-ene (3a). Compounds 10 and 11 are usually isolated from lichen, hence they are possible contaminants of lichen harvested with the bark. Compounds 1, 3a, 5-14 were not significantly cytotoxic to the primary epidermal melanocyte cells (P > 0.05) when compared to the negative and positive controls (DMSO, 0.1% and hydrogen peroxide, 30 wt% in water). Inhibition of tyrosinase was significantly greater with respect to the negative control (P < 0.001) for compounds 3a, 5-8 and 9-10 at 10 muM and for compounds 5-8 and 9-10 at 100 muM. Compared to hydroquinone (the positive control) at 10 muM, the level of inhibition was comparable or to that of compounds 3a, 5, 6, and 8-10 at 10 muM, with 9 and 10 showing a greater level of inhibition. Inhibition of melanin was both concentration and time dependent for all compounds tested with higher melanin content at 24 h compared to 48 h s and at 10 mM compared to100 mM at both time points; melanin content was significantly lower for hydroquinone at both time points and concentrations. CONCLUSIONS: Compounds 1, 5-14, isolated from Cassipourea flanaganii and the derivative 3a showed low cytotoxicity. All compounds had a clear time and concentration dependent effect on melanin content which did not appear to be dependent on their inhibition of tyrosinase.
Chemical and Genetic Study of two Ligularia Hybrids in Shangrila County, Yunnan Province, China.[Pubmed:30725554]
Nat Prod Commun. 2016 Aug;11(8):1057-1060.
The composition of root chemicals was examined for samples of L. cyathiceps x L. duciformis, L. duciformis x L. yunnanensis, and L. yunnanensis. Various furanoeremophilanes were isolated from a sample of L. cyathiceps x L. duciformis and found to be very similar to those isolated from L. cyathiceps. Lupeol and Docosyl ferulate were isolated from L. yunnanensis, L. duciformis, and L. duciformis x L. yunnanensis.
Amelioration of testosterone induced benign prostatic hyperplasia by Prunus species.[Pubmed:27235020]
J Ethnopharmacol. 2016 Aug 22;190:33-45.
ETHNOPHARMACOLOGICAL RELEVANCE: Benign prostatic hyperplasia (BPH) is a common urological disorder of men. The ethnomedicinal use of an African plant Prunus africana (Hook.f.) Kalkman (Pygeum) in treating men's problems made it a popular remedy all over the globe for the treatment of BPH and related disorders. However, rampant collections made from the wild in Africa have pushed the plant to Appendix II of CITES demanding conservation of the species. AIM OF THE STUDY: In the present study, the aim was to unearth the protective effect of bark of different species of Prunus against BPH. The five selected Indian plants of family Rosaceae viz. Prunus amygdalus Stokes, Prunus armeniaca L., Prunus cerasoides Buch.-Ham. ex D. Don, Prunus domestica L. and Prunus persica (L.) Batsch were evaluated against P. africana (Hook.f.) Kalkman for a suitable comparison of efficacy as antiBPH agents. MATERIALS AND METHODS: The antiBPH activity was evaluated in testosterone (2mg/kg/day, s.c, 21 days) induced BPH in Wistar rats. The parameters studied were body weights; histopathological examination, immunohistochemistry (PCNA) and biochemical estimations of the prostate; supported by prostatic index, testicular index, creatinine, testosterone levels; antioxidant and anti-inflammatory evaluation. The study also included chemical profiling using three markers (beta-sitosterol, Docosyl ferulate and ursolic acid) and estimation of beta-sitosterol content through GC. RESULTS: The Prunus species showed the presence of all the three markers in their TLC fingerprint profile and maximum amount of beta-sitosterol by GC was observed in P. domestica. Interestingly, all the species exhibited significant amelioration in testosterone induced parameters with P. domestica showing the most encouraging effect as indicated from histopathological examination, immunohistochemistry and biochemical studies. The Prunus species further showed remarkable anti-inflammatory and antioxidant activity signifying their role in interfering with various possible factors involved in BPH. CONCLUSIONS: These findings are suggestive of a meaningful inhibitory effect of testosterone induced BPH by the bark of different species of Prunus in the order of P. domestica, P. persica, P. amygdalus, P. cerasoides and P. armeniaca with an efficacy of P. domestica comparable to P. africana and can be used as the potential backup of Pygeum for the management of BPH.
Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans.[Pubmed:25256822]
Phytochemistry. 2014 Dec;108:177-88.
Three diterpenoids, 1-oxomicrostegiol (1), viroxocin (2), viridoquinone (3), were isolated from the roots of Salvia viridis L. cvar. Blue Jeans. Five known diterpenoids, microstegiol (4), 7alpha-acetoxy-14-hydroxy-8,13-abietadiene-11,12-dione (5; 7-O-acetylhorminone tautomer), 7alpha,14-dihydroxy-8,13-abietadiene-11,12-dione (6; horminone tautomer), ferruginol and salvinolonyl 12-methyl ether (7) were also found in the roots together with 1-Docosyl ferulate (8), and a mixture of 2-(4'-alkoxyphenyl) ethyl alkanoates (9). Two lupane triterpenoids, 2alpha-acetoxy-lup-20(29)-en-3beta-ol (10), and 3beta-acetoxy-lup-20(29)-en-2alpha-ol (11) were found in the aerial parts together with known compounds, lup-20(29)-ene-2alpha,3beta-diol (12), ursolic acid, oleanolic acid, beta-sitosterol and beta-sitosterol glucoside. A known phenylpropanoid, trans-verbascoside (or acteoside; 13), was the main constituent in the polar fraction of the aerial part, and it is now reported in the genus Salvia for the first time. Other polyphenolic compounds were cis-verbascoside (14), leucosceptoside A (15), martynoside (16), caffeic acid, 6-O-caffeoyl-glucose (18), rosmarinic acid, salidroside, luteolin-7-O-alpha-rhamnopyranosyl-(1-->6)-beta-galactopyranoside, luteolin-7-O-beta-galactopyranoside, luteolin-7-O-alpha-rhamnopyranosyl-(1-->6)-beta-glucopyranoside, luteolin-7-O-beta-glucopyranoside, and apigenin-7-O-beta-glucopyranoside. The structures were determined by 1D-, 2D-NMR and HR-ESI-MS techniques. Compounds 6, 10, ferruginol, ursolic acid and oleanolic acid exhibited antibacterial activity against Enterococcus faecalis (ATCC 775) with MIC 50 muM, 25 muM, 50 muM, 12.5 muM, 12.5 muM respectively. Ferruginol, ursolic acid and oleanolic acid were also active against Staphylococcus aureus (ATCC 6571), and Bacillus cereus (ATCC 2599) with MIC 12.5-50 muM. 4 was also active against S.aureus (ATCC 6571) with MIC 50 muM. These values are consistent with previous studies on the antimicrobial activity of Salvia diterpenoids.
[Chemical constituents from tubers of Dioscorea bulbifera].[Pubmed:19873780]
Zhongguo Zhong Yao Za Zhi. 2009 Jul;34(13):1679-82.
OBJECTIVE: To study the chemical constituents in the tubers of Dioscorea bulbifera. METHOD: Compounds were isolated and purified with silica gel, ODS and Sephadex LH-20 column chromatography, their structures were determined by using spectroscopic methods including MS and NMR. RESULT: Fourteen compounds were isolated and identified as stigmasterol (1), mono-arachidin (2), 1,7-bis-(4-hydroxyphenyl)-1E,4E,6E-heptatrien-3-one (3), behenic acid (4), demethyl batatasin IV (5), 2,3'-di-hydroxy-4',5'-dimethoxybibenzyl (6), diosbulbin B (7), diosbulbin D (8), Docosyl ferulate (9), 7-bis-(4-hydroxyphenyl) -4E, 6E-heptadien-3-one (10), 5,3,4-trihydroxy-3,7-dimethoxyflavone (11), tristin(12), protocatechuic acid (13), adenosine (14). CONCLUSION: Compounds 24, 6, 9, 10, 12, 14 were isolated from the genus Dioscorea for the first time.
[Study on chemical constituents in roots and rhizomes of Ligularia duciforms II].[Pubmed:18652347]
Zhongguo Zhong Yao Za Zhi. 2008 May;33(9):1018-20.
OBJECTIVE: To study the chemical constituents in roots and rhizomes of Ligularia duciforms. METHOD: 90% ethanol extract was isolated and purified by silica gel and sephadex LH-20 column chromatography, the structures of compounds were identified by physicochemical properties and spectral analysis. RESULT: Nine compounds were isolated and identified as lupeol (1), isoscopoletin (2), isoline (3), duciformine (4), (2S, 3S, 4R)-sphinganine-(2'R)-delta5',6' (E)-2'-hydroxytetracosanoylamino (5), tetracosanoic acid (6), tetracosanoicacid glyceride (7), (E)-Docosyl ferulate (8), (E)-docosyl caffeate (9). CONCLUSION: Compounds 1, 2, 5-7 were isolated for the first time from this plant, and the compound 2, 5-7 were isolated firstly from the genus Ligularia.
[Studies on chemical constituents in herb of Centella asiatica].[Pubmed:17802882]
Zhongguo Zhong Yao Za Zhi. 2007 Jun;32(12):1182-4.
OBJECTIVE: To study the chemical constituents from Centella asiatica. METHOD: Chemical constituents were isolated by repeated column chromatography (Toyopearl HW-40C and HPLC) and their structures were elucidated on the basis of spectroscopic method. RESULT: Five compounds were identified as: Docosyl ferulates (1), bayogenin (2), 3beta-6beta-23-trihydroxyolean-12-en-28-oic acid (3), 3beta-6beta-23-trihydroxyurs-12-en-28-oic acid (4), D-gulonic acid (5). CONCLUSION: All of the Compounds were isolated for the first time from C. asiatica.
[Studies on chemical constituents of Tinospora hainanesis].[Pubmed:12017002]
Yao Xue Xue Bao. 1998 May;33(5):350-4.
Tinospora hainanesis is a new species of Menispermaceae plant. It is used as folk remedy for joint pain and physical injury. Five compounds were isolated from the vine stalk of Tinospora hainanesis. By spectral analysis and chemical methods, the structures of the compounds were identified as makisterone A (I), 2,3-dimethoxy-9,10-dihydroxy-N-methyltetrahydroproto-berberine quaternary salt (II), palmatine (III), beta-amyrin (IV) and Docosyl ferulate (V). II is a new quaternary alkaloid named as haitinosporine. I, IV and V were isolated for the first time from the plants of Tinospora genus.
[Determination of docosyl ferulate in extract of Pygeum africanton Hook. by high performance liquid chromatography (HPLC)].[Pubmed:15739376]
Se Pu. 1997 May;15(3):259-60.
This paper reports a method for the determination of Docosyl ferulate in the extract of bark of pygeum africanum Hook. by HPLC. After the sample was pretreated, the Docosyl ferulate was well separated and determined on a Spherisorb C18 column (250 x 4.6mm, 5microm) using a mobile phase of methanol with a flow rate of 1mL/min. The column temperature was selected at 40 degrees C to avoid tailing of peak. UV detection was performed at 326nm. In order to confirm the Docosyl ferulate separated from sample, the peak apex at 10.4 minute was scanned from 195nm to 360nm by photodiode array detector. Its spectrum showed the maxium absorption peak at 240nm and 326nm corresponding with the spectrum of Docosyl ferulate. The linear correlation was observed from the 10mg/L to 100mg/L of Docosyl ferulate (r = 0.9995). The average recovery was 98.4% +/- 1.98%. Three batches of sample were determined.


