EuphorbetinCAS# 35897-99-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35897-99-5 | SDF | Download SDF |
| PubChem ID | 5317297.0 | Appearance | Powder |
| Formula | C18H10O8 | M.Wt | 354.27 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
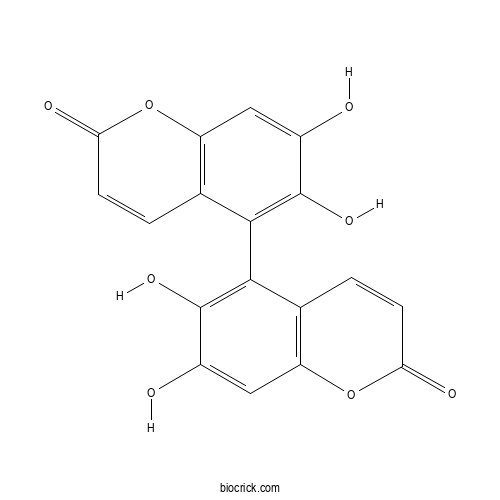
| Chemical Name | 5-(6,7-dihydroxy-2-oxochromen-5-yl)-6,7-dihydroxychromen-2-one | ||
| SMILES | C1=CC(=O)OC2=C1C(=C(C(=C2)O)O)C3=C(C(=CC4=C3C=CC(=O)O4)O)O | ||
| Standard InChIKey | MFAPGDLENWJYSK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H10O8/c19-9-5-11-7(1-3-13(21)25-11)15(17(9)23)16-8-2-4-14(22)26-12(8)6-10(20)18(16)24/h1-6,19-20,23-24H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Euphorbetin Dilution Calculator

Euphorbetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8227 mL | 14.1135 mL | 28.2271 mL | 56.4541 mL | 70.5676 mL |
| 5 mM | 0.5645 mL | 2.8227 mL | 5.6454 mL | 11.2908 mL | 14.1135 mL |
| 10 mM | 0.2823 mL | 1.4114 mL | 2.8227 mL | 5.6454 mL | 7.0568 mL |
| 50 mM | 0.0565 mL | 0.2823 mL | 0.5645 mL | 1.1291 mL | 1.4114 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2823 mL | 0.5645 mL | 0.7057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hericene A
Catalog No.:BCX1005
CAS No.:157207-54-0
- Hericene B
Catalog No.:BCX1004
CAS No.:157207-55-1
- α-Glucosyl Hesperidin
Catalog No.:BCX1003
CAS No.:161713-86-6
- Neohesperidose
Catalog No.:BCX1002
CAS No.:17074-02-1
- Ovatodiolide
Catalog No.:BCX1001
CAS No.:3484-37-5
- Anisomelic acid
Catalog No.:BCX1000
CAS No.:59632-76-7
- Apigenin triacetate
Catalog No.:BCX0999
CAS No.:3316-46-9
- Homodihydrocapsaicin II
Catalog No.:BCX0998
CAS No.:71239-21-9
- Neopuerarin B
Catalog No.:BCX0997
CAS No.:1150314-39-8
- Hericene D
Catalog No.:BCX0996
CAS No.:1343477-87-1
- Maltononaose
Catalog No.:BCX0995
CAS No.:6471-60-9
- Maltodecaose
Catalog No.:BCX0994
CAS No.:6082-21-9
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
- Isomaltoheptaose
Catalog No.:BCX1009
CAS No.:6513-12-8
- Isomaltohexaose
Catalog No.:BCX1010
CAS No.:6175-02-6
- Isomaltopentaose
Catalog No.:BCX1011
CAS No.:6082-32-2
- Isomaltotetraose
Catalog No.:BCX1012
CAS No.:35997-20-7
- 6'-Hydroxy-3,4,2',3',4'-pentamethoxychalcone
Catalog No.:BCX1013
CAS No.:114021-62-4
- Cycloastragenol-6-O-β-D-glucoside
Catalog No.:BCX1014
CAS No.:86764-12-7
- 6,8-Dihydroxy-1,2,7-trimethoxy-3-methylanthraquinone
Catalog No.:BCX1015
CAS No.:1622982-59-5
- Zeatin
Catalog No.:BCX1016
CAS No.:13114-27-7
- Neopuerarin A
Catalog No.:BCX1017
CAS No.:1150314-34-3
- Docosyl ferulate
Catalog No.:BCX1018
CAS No.:101927-24-6
Colon cancer therapy with calcium phosphate nanoparticles loading bioactive compounds from Euphorbia lathyris: In vitro and in vivo assay.[Pubmed:36156367]
Biomed Pharmacother. 2022 Nov;155:113723.
Amorphous calcium phosphate nanoparticles (ACP NPs) exhibit excellent biocompatibility and biodegradability properties. ACP NPs were functionalized with two coumarin compounds (esculetin and Euphorbetin) extracted from Euphorbia lathyris seeds (BC-ACP NPs) showing high loading capacity (0.03% and 0.34% (w/w) for esculetin and Euphorbetin, respectively) and adsorption efficiency (2.6% and 33.5%, respectively). BC-ACP NPs, no toxic to human blood cells, showed a more selective cytotoxicity against colorectal cancer (CRC) cells (T-84 cells) (IC50, 71.42 microg/ml) compared to non-tumor (CCD18) cells (IC50, 420.77 microg/ml). Both, the inhibition of carbonic anhydrase and autophagic cell death appeared to be involved in their action mechanism. Interestingly, in vivo treatment with BC-ACPs NPs using two different models of CRC induction showed a significant reduction in tumor volume (62%) and a significant decrease in the number and size of polyps. A poor development of tumor vasculature and invasion of normal tissue were also observed. Moreover, treatment increased the bacterial population of Akkermansia by restoring antioxidant systems in the colonic mucosa of mice. These results show a promising pathway to design innovative and more efficient therapies against CRC based on biomimetic calcium phosphate NPs loaded with natural products.
Phytophenol Dimerization Reaction: From Basic Rules to Diastereoselectivity and Beyond.[Pubmed:35956790]
Molecules. 2022 Jul 28;27(15):4842.
Phytophenol dimerization, which is a radical-mediated coupling reaction, plays a critical role in many fields, including lignin biosynthesis. To understand the reaction, 2,2-diphenyl-1-picrylhydrazyl radical was used to initiate a series of phytophenol dimerization reactions in methanol. The products were identified using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF-MS/MS) analysis in situ. The identified products mainly included biphenols, magnolol, honokiol, gingerol 6,6'-dimers, 3,6-dimethoxylcatechol beta,beta' dimer, Euphorbetin, bis-eugenol, dehydrodiisoeugenol, trans-epsilon-viniferin, (+) pinoresinol, and (-) pinoresinol. Structure-function relationship analysis allowed four basic rules to be defined: meta-excluded, C-C bonding domination, ortho-diOH co-activation, and exocyclic C=C involvement. The exocyclic C=C involvement, however, required conjugation with the phenolic core and the para-site of the -OH group, to yield a furan-fused dimer with two chiral centers. Computational chemistry indicated that the entire process was completed via a radical coupling reaction and an intramolecular conjugate addition reaction. Similar results were also found for the horseradish peroxidase (HRP)-catalyzed coniferyl alcohol dimerization, which produced (+) and (-) pinoresinols (but no (-) epipinoresinol), suggesting that the HRP-catalyzed process was essentially an exocyclic C=C-involved phytophenol dimerization reaction. The reaction was highly diastereoselective. This was attributed to the intramolecular reaction, which prohibited Re-attack. The four basic rules and diastereoselectivity can explain and even predict the main products in various chemical and biological events, especially oxidase-catalyzed lignin cyclization.
Potential phytochemical inhibitors of SARS-CoV-2 helicase Nsp13: a molecular docking and dynamic simulation study.[Pubmed:34117992]
Mol Divers. 2022 Feb;26(1):429-442.
The SARS-CoV-2 helicase Nsp13 is a promising target for developing anti-COVID drugs. In the present study, we have identified potential natural product inhibitors of SARS-CoV-2 Nsp13 targeting the ATP-binding site using molecular docking and molecular dynamics (MD) simulations. MD simulation of the prepared crystal structure of SARS-CoV-2 Nsp13 was performed to generate an ensemble of structures of helicase Nsp13 capturing the conformational diversity of the ATP-binding site. A natural product library of more than 14,000 phytochemicals from Indian medicinal plants was used to perform virtual screening against the ensemble of Nsp13 structures. Subsequently, a two-stage filter, first based on protein-ligand docking binding energy value and second based on protein residues in the ligand-binding site and non-covalent interactions between the protein residues and the ligand in the best-docked pose, was used to identify 368 phytochemicals as potential inhibitors of SARS-CoV-2 helicase Nsp13. MD simulations of the top inhibitors complexed with protein were performed to confirm stable binding, and to compute MM-PBSA based binding energy. From among the 368 potential phytochemical inhibitors, the top identified potential inhibitors of SARS-CoV-2 helicase Nsp13 namely, Picrasidine M, (+)-Epiexcelsin, Isorhoeadine, Euphorbetin and Picrasidine N, can be taken up initially for experimental studies.
Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer.[Pubmed:33572111]
Nutrients. 2021 Feb 9;13(2):566.
The seeds of Euphorbia lathyris have been used in traditional medicine to treat various medical conditions. However, neither all of their active biocompounds nor the molecular mechanisms underlying their therapeutic effects have been described. A new ethanolic extract of defatted flour from mature seeds of Euphorbia lathyris showed a high total polyphenol content and significant antioxidant activity. Chromatographic analysis showed that esculetin, Euphorbetin, gaultherin, and kaempferol-3-rutinoside were the most abundant polyphenolic bioactive compounds. Antiproliferative assays showed a high and selective antitumor activity against colon cancer cell lines (T84 and HCT-15). In addition, a significant antiproliferative activity against glioblastoma multiforme cells was also demonstrated. Its mechanism of action to induce cell death was mediated by the overexpression of caspases 9, 3, and 8, and by activation of autophagy. Interestingly, a reduction in the migration capacity of colon cancer cells and a significant antiangiogenic effect on human umbilical vein endothelial cells were also demonstrated. Finally, the extract significantly reduced the subpopulations of cancer stem cells. This extract could be the basis to develop new therapeutic strategies for the treatment of colon cancer, although further experiments will be necessary to determine its in vivo effects.
Chemical Constituents from Fraxinus hupehensis and Their Antifungal and Herbicidal Activities.[Pubmed:31906487]
Biomolecules. 2020 Jan 2;10(1):74.
The phytochemical investigation of Fraxinus hupehensis led to the isolation and characterization of ten compounds which were identified as fraxin (1), fraxetin (2), esculetin (3), cichoriin (4), Euphorbetin (5), kaempferol-3-O-beta-rutinoside (6), oleuropein (7), linoleic acid (8), methyl linoleate (9), and beta-sitosterol (10). Structures of the isolated constituents were characterized by (1)H NMR, (13)C NMR and HRMS. All the compounds, except compounds 3 and 4, were isolated for the first time from this plant. Further, this was the first report for the occurrence of compound 5 in the Fraxinus species. Antifungal activity evaluation showed that compound 2 exhibited significant inhibitory effects against Bipolaris maydis, Sclerotium rolfsii, and Alternaria solani with EC(50) values of 0.31 +/- 0.01 mmol/L, 10.50 +/- 0.02 mmol/L, and 0.40 +/- 0.02 mmol/L respectively, compared to the positive control, Carbendazim, with its EC(50) values of 0.74 +/- 0.01 mmol/L, 1.78 +/- 0.01 mmol/L and 1.41 +/- 0.00 mmol/L. Herbicidal activity tests showed that compounds 8-10 had strong inhibitory effects against the roots of Echinochloa crus-galli with EC(50) values of 1.16 +/- 0.23 mmol/L, 1.28 +/- 0.58 mmol/L and 1.33 +/- 0.35 mmol/L respectively, more potently active than that of the positive control, Cyanazine, with its EC(50) values of 1.56 +/- 0.44 mmol/L. However, none of the compounds proved to be active against the tested bacteria (Erwinia carotovora, Pseudomonas syringae, and Ralstonia solanacearum).
[LC/MS/MS Analysis of the Metabolites of Lathyrane Diterpenoids in Caco-2 Cells].[Pubmed:30204381]
Zhong Yao Cai. 2016 Aug;39(8):1771-4.
OBJECTIVE: To identify the metabolites of Euphorbetin L1,Euphorbetin L2,Euphorbetin L8 and 6( 17),12( E)-lathyrol-5,15-diacetate-3-phenylacetate in Caco-2 cells by LC/MS/MS. METHODS: Caco-2 cells were cultured with 100 mug/mol lathyrane diterpenoid for 3,6,12 h,respectively. Then the samples were collected,purified and identified by LC/MS/MS. RESULTS: The major metabolites of Euphorbetin L1 were two methylated products which were obtained after hydrolysis of the ester. The major metabolites of Euphorbetin L2,Euphorbetin L8 and 6( 17),12( E)-lathyrol-5,15-diacetate-3-phenylacetate were hydrolysis products of the ester. CONCLUSION: The main metabolic pathway of Euphorbetin L1 is methylation and hydrolysis of the ester. The main metabolic pathway of Euphorbetin L2,Euphorbetin L8 and 6( 17),12( E)-lathyrol-5,15-diacetate-3-phenylacetate is hydrolysis of the ester. LC/MS/MS can identify the metabolites of Euphorbetin L1,Euphorbetin L2,Euphorbetin L8 and 6( 17),12( E)-lathyrol-5,15-diacetate-3-phenylacetate in Caco-2 cells quickly and sensitively.
A new dicoumarin and anticoagulant activity from Viola yedoensis Makino.[Pubmed:19306914]
Fitoterapia. 2009 Jul;80(5):283-5.
A new dicoumarin, named as dimeresculetin (1), together with another dicoumarin, Euphorbetin (2) and esculetin (3) were isolated from the ethyl acetate extract of the dried whole plants of Viola yedoensis Makino. The structure of 1 was elucidated as 7-hydroxy-6-[(6,7-dihydroxy-2-oxo-2H-1-benzopyran-5-yl)oxy]-2H-1-benzopyran-2-one on the basis of extensive NMR, as well as the other spectral analysis. Compounds 1-3 exhibited anticoagulant activities with respect to activated partial thromboplastin time (APTT), prothrombin time (PT) and thrombin time (TT).


