NeohesperidoseCAS# 17074-02-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17074-02-1 | SDF | Download SDF |
| PubChem ID | 5218787.0 | Appearance | Powder |
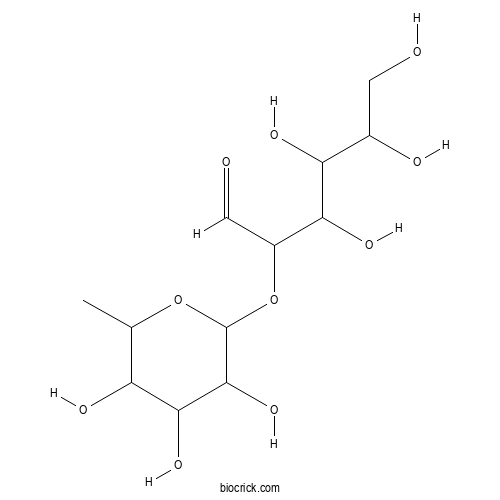
| Formula | C12H22O10 | M.Wt | 326.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,4,5,6-tetrahydroxy-2-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyhexanal | ||
| SMILES | CC1C(C(C(C(O1)OC(C=O)C(C(C(CO)O)O)O)O)O)O | ||
| Standard InChIKey | BQEBASLZIGFWEU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H22O10/c1-4-7(16)10(19)11(20)12(21-4)22-6(3-14)9(18)8(17)5(15)2-13/h3-13,15-20H,2H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Neohesperidose Dilution Calculator

Neohesperidose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0647 mL | 15.3233 mL | 30.6466 mL | 61.2933 mL | 76.6166 mL |
| 5 mM | 0.6129 mL | 3.0647 mL | 6.1293 mL | 12.2587 mL | 15.3233 mL |
| 10 mM | 0.3065 mL | 1.5323 mL | 3.0647 mL | 6.1293 mL | 7.6617 mL |
| 50 mM | 0.0613 mL | 0.3065 mL | 0.6129 mL | 1.2259 mL | 1.5323 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3065 mL | 0.6129 mL | 0.7662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ovatodiolide
Catalog No.:BCX1001
CAS No.:3484-37-5
- Anisomelic acid
Catalog No.:BCX1000
CAS No.:59632-76-7
- Apigenin triacetate
Catalog No.:BCX0999
CAS No.:3316-46-9
- Homodihydrocapsaicin II
Catalog No.:BCX0998
CAS No.:71239-21-9
- Neopuerarin B
Catalog No.:BCX0997
CAS No.:1150314-39-8
- Hericene D
Catalog No.:BCX0996
CAS No.:1343477-87-1
- Maltononaose
Catalog No.:BCX0995
CAS No.:6471-60-9
- Maltodecaose
Catalog No.:BCX0994
CAS No.:6082-21-9
- 1,3-Disinapoylglucose
Catalog No.:BCX0993
CAS No.:1423128-15-7
- S-Isogambogic acid
Catalog No.:BCX0992
CAS No.:942623-57-6
- Forbesione
Catalog No.:BCX0991
CAS No.:180961-63-1
- (9Z,11E)-13-Oxo-9,11-octadecadienoic Acid
Catalog No.:BCX0990
CAS No.:54739-30-9
- α-Glucosyl Hesperidin
Catalog No.:BCX1003
CAS No.:161713-86-6
- Hericene B
Catalog No.:BCX1004
CAS No.:157207-55-1
- Hericene A
Catalog No.:BCX1005
CAS No.:157207-54-0
- Euphorbetin
Catalog No.:BCX1006
CAS No.:35897-99-5
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
- Isomaltoheptaose
Catalog No.:BCX1009
CAS No.:6513-12-8
- Isomaltohexaose
Catalog No.:BCX1010
CAS No.:6175-02-6
- Isomaltopentaose
Catalog No.:BCX1011
CAS No.:6082-32-2
- Isomaltotetraose
Catalog No.:BCX1012
CAS No.:35997-20-7
- 6'-Hydroxy-3,4,2',3',4'-pentamethoxychalcone
Catalog No.:BCX1013
CAS No.:114021-62-4
- Cycloastragenol-6-O-β-D-glucoside
Catalog No.:BCX1014
CAS No.:86764-12-7
Structural Characterization of Peripolin and Study of Antioxidant Activity of HMG Flavonoids from Bergamot Fruit.[Pubmed:36290571]
Antioxidants (Basel). 2022 Sep 20;11(10):1847.
The structural characterization of a new flavonoid from bergamot fruit (Citrus bergamia Risso) carrying the 3-hydroxy-3-methyl glutaryl (HMG) ester moiety has been accomplished, and its antioxidant ability was tested from a chemical point of view. The peculiarity of the new molecule, named peripolin, relies on the presence of the HMG chemical group linked to the sugar portion of neoeriocitrin; the structure was elucidated using both high-resolution mass spectrometry and nuclear magnetic resonance experiments performed on the purified molecule extracted from the fruit. The antioxidant ability of the new molecule was tested by classical chemical approaches, such as DPPH, ABTS and FRAP assays, and from a theoretical point of view. (1)H and (13)C NMR experiments and HR-ESI-MS/MS experiments show unequivocally that the HMG moiety is linked to the primary position of the glucose unit of Neohesperidose, while the chemical tests and the computational results show that peripolin possesses strong antioxidant behavior, similar to that of neoeriocitrin and remarkably higher respect to the other flavonoids present in the fruit. Furthermore, the quantitative assays carried out by UPLC-MS/MS showed that its amount in the fruit is similar to that of the other main flavonoids. Furthermore, molecular dynamics simulations allowed us to investigate the possible conformations adopted by the antioxidants in the presence of water molecules. In particular, the switch of open-closed conformations of HMG-containing species was evidenced. As far as the reaction with DPPH, the calculation of DeltaG(rea) supported the experimental outcomes regarding the peripolin and neoeriocitrin activity. In conclusion, bergamot fruit, already known for its potential to lower the level of blood cholesterol, has been proven to contain molecules such as neoeriocitrin and the newly characterized peripolin, which could have important in-vivo antioxidant characteristics.
Neutral loss scan in complement with high-resolution MS/MS: Combination of detection methods for flavonoid and limonoid glycosides analysis.[Pubmed:35088488]
J Mass Spectrom. 2022 Jan 10;57(2):e4810.
In this study, neutral loss scan and high-resolution MS/MS were used in combination to detect and tentatively identify various flavonoid and limonoid glycosides in navel orange albedo, juice, peel and pulp. These compound classes are of research interest due to their flavour and bioactive properties, and although flavonoid glycosides have been previously studied in other food matrices, to the best of our knowledge, neutral loss scans have not been used for the elucidation of limonoid glycosides. Neutral loss masses of 120, 162 and 308 Da were selected for the detection of hexose, rutinose and Neohesperidose-substituted flavonoids, whereas 197 Da was explored for limonoid glycosides due to their tendency to form ammonium adducts. Fragmentation patterns obtained from targeted MS/MS were then used to differentiate rutinose and Neohesperidose substituents as well as flavonoid subclasses of flavones, flavanones and flavonols. Additionally, high-resolution MS/MS was also used for the identification of aglycones by accurate mass (to four decimal places), allowing for the differentiation of aglycones with similar unit masses but different chemical formulas. In total, 19 flavonoid glycosides and six limonoid glycosides were detected. This workflow allows for a rapid screening of flavonoid and limonoid glycosides in citrus, which can be further extended to other food products such as tea.
Phytochemical characteristics of bergamot oranges from the Ionian islands of Greece: A multi-analytical approach with emphasis in the distribution of neohesperidose flavanones.[Pubmed:33131954]
Food Chem. 2021 May 1;343:128400.
The present study describes the peculiar phytochemical characteristics of bergamots cultivated in distinct islands of the Ionian Sea. Ultrahigh-performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS) supported by 1 and 2D NMR spectroscopy was used for unambiguous metabolic profiling of albedo, flavedo and juice samples. Profile differences were determined using a multi-analytical clustering approach based on high-performance thin-layer chromatography fingerprints and UHPLC-HRMS data. Finally, a validated HPLC method offering good precision (0.12-0.94%) and accuracy (95.25-103.93%) was proposed for the quantification of the major flavanones. A total of 37 secondary metabolites were characterized belonging to flavonoids, limonoids and coumarins. Their distribution was tissue-dependent and varied significantly from bergamots of other geographical locations. Surprisingly, neoeriocitrin was the major flavanone, reaching 1.69 +/- 0.05 g/L in the juice and 5.24 +/- 0.12 mg/g in albedo. This is the highest reported amount among Citrus species, rendering Ionian bergamots a promising candidate for novel functional products.
Glucosylation of flavonoids and flavonoid glycosides by mutant dextransucrase from Lactobacillus reuteri TMW 1.106.[Pubmed:31325545]
Carbohydr Res. 2019 Sep 1;483:107741.
Flavonoids are commonly abundant, plant-derived polyphenolic compounds which are responsible for color, taste, and antioxidant properties of certain plant based foods. Glucosylation by glucansucrases or other glycosyltransferases/glycoside hydrolases has been described to be a promising approach to modify stability, solubility, bioavailability, and taste profile of flavonoids and other compounds. In this study, we modified and applied a recombinant dextransucrase from Lactobacillus reuteri TMW 1.106 to glucosylate various flavonoids and flavonoid glycosides. The glucoconjugates were subsequently isolated and characterized by using two-dimensional NMR spectroscopy. Efficient glucosylation was achieved for quercetin and its glycosides quercetin-3-O-beta-glucoside and rutin. Significant portions of alpha-glucose conjugates were also obtained for epigallocatechin gallate, dihydromyricetin, and cyanidin-3-O-beta-glucoside, whereas glucosylation efficiency was low for naringin and neohesperidin dihydrochalcone. Most of the flavonoids with a catechol or pyrogallol group at the B-ring were predominantly glucosylated at position O4'. However, glycosyl substituents such as beta-glucose, rutinose, or Neohesperidose were glucosylated at varying positions. Therefore, mutant dextransucrase from L. reuteri TMW 1.106 can be applied for versatile structural modification of flavonoids.
Synthesis of trilobatin from naringin via prunin as the key intermediate: acidic hydrolysis of the alpha-rhamnosidic linkage in naringin under improved conditions.[Pubmed:29865928]
Biosci Biotechnol Biochem. 2018 Sep;82(9):1463-1467.
Trilobatin [4'-(beta-D-glucopyranosyloxy)-2',4",6'-trihydroxydihydrochalcone] was synthesized from commercially available naringin in three steps with an overall yield of 30%. The key step was the acid-catalyzed site-selective hydrolysis of terminal alpha-rhamnopyranosidic linkage in Neohesperidose involved in naringin under controlled conditions, by applying a high-pressure steam sterilizer.
Functional Characterization of a Flavonoid Glycosyltransferase in Sweet Orange (Citrus sinensis).[Pubmed:29497429]
Front Plant Sci. 2018 Feb 15;9:166.
Fruits of sweet orange (Citrus sinensis), a popular commercial Citrus species, contain high concentrations of flavonoids beneficial to human health. These fruits predominantly accumulate O-glycosylated flavonoids, in which the disaccharides [Neohesperidose (rhamnosyl-alpha-1,2-glucose) or rutinose (rhamnosyl-alpha-1,6-glucose)] are linked to the flavonoid aglycones through the 3- or 7-hydroxyl sites. The biotransformation of the flavonoid aglycones into O-rutinosides or O-neohesperidosides in the Citrus plants usually consists of two glycosylation reactions involving a series of uridine diphosphate-sugar dependent glycosyltransferases (UGTs). Although several genes encoding flavonoid UGTs have been functionally characterized in the Citrus plants, full elucidation of the flavonoid glycosylation process remains elusive. Based on the available genomic and transcriptome data, we isolated a UGT with a high expression level in the sweet orange fruits that possibly encodes a flavonoid glucosyltransferase and/or rhamnosyltransferase. Biochemical analyses revealed that a broad range of flavonoid substrates could be glucosylated at their 3- and/or 7-hydrogen sites by the recombinant enzyme, including hesperetin, naringenin, diosmetin, quercetin, and kaempferol. Furthermore, overexpression of the gene could significantly increase the accumulations of quercetin 7-O-rhamnoside, quercetin 7-O-glucoside, and kaempferol 7-O-glucoside, implying that the enzyme has flavonoid 7-O-glucosyltransferase and 7-O-rhamnosyltransferase activities in vivo.
Targeting structural motifs of flavonoid diglycosides using collision-induced dissociation experiments on flavonoid/Pb2+ complexes.[Pubmed:23221119]
Eur J Mass Spectrom (Chichester). 2012;18(5):465-73.
Differentiation of flavonoid congeners remains a challenging analytical problem and confirming the structures of the different isomers is difficult, even when they can be adequately separated from mixtures. In the present report, in order to overcome the limits of our recently proposed method that relies on the distinctive CID behaviors of [(flavonoid - H(+)) + Cu(2+)] complexes to obtain direct structural evidences, we decided to investigate the possibility of using Pb(II) complexation to generate significant differences upon CID. We selected five flavonoid diglycosides with targeted structural features to estimate the applicability of this methodology. Electrospray ionization from methanol-not acetonitrile-solutions was advantageously used for preparing the [(flavonoid diglycoside - H(+)) + Pb(2)+](+) complexes. Upon collisional activation, [(flavonoid diglycoside- H(+)) + Pb(2+)](+) ions mainly dissociate by glycosidic bond cleavage. Nevertheless, specific cross-ring cleavages are also induced and lead to a clear-cut determination of (i) the nature of the disaccharide group, i.e. rutinose or Neohesperidose, (ii) the nature of the aglycone part, flavanone or flavone and (iii) the relative position of the disaccharide substituent on the aglycone part, i.e. 3-O- vs 7-O positions.
Screening and identification of disaccharides with insulin mimetic activity against L6 cells.[Pubmed:22484948]
Biosci Biotechnol Biochem. 2012;76(4):841-2.
Insulin mimetics are considered as prospective anti-diabetic agents, and the disaccharide, Neohesperidose, has been found to show insulin mimetic activity against L6 cells. We screened several other disaccharides for their insulin mimetic activity and identified three new insulin mimetic disaccharides.
Characteristic fragmentation patterns of trimethylsilyl and trimethylsilyl-oxime derivatives of plant disaccharides as obtained by gas chromatography coupled to ion-trap mass spectrometry.[Pubmed:21924428]
J Chromatogr A. 2011 Oct 28;1218(43):7864-8.
The characteristic fragmentation pattern of six reducing and two non reducing type disaccharides-(Neohesperidose, acuminose, sambubiose, rutinose, vicianose, primverose, and two arabinosyl-inositols) has been described. These saccharides have not been previously identified by on-line chromatographic techniques. Unambiguous specific characteristics of the TMS (oxime)s such as mass distribution, syn/anti oximes ratios and elution order proved to be associated with their reducing or non reducing character, with their aldosyl property and with the position of their O-glycosidic linkages. The practical utility of the mass fragmentation study of these rare disaccharides was demonstrated, at the first time, by the simultaneous, on-line identification and quantification of the acuminose, vicianose, primverose and arabinosyl-inositol contents of tea leaves, from green and black tea blends of Indian and Chinese origin.
Study of kaempferol glycoside as an insulin mimic reveals glycon to be the key active structure.[Pubmed:24900249]
ACS Med Chem Lett. 2010 Oct 11;2(1):17-21.
Diabetes mellitus is increasing in prevalence with patient numbers rising throughout the world. Current treatments for diabetes mellitus focus on control of blood glucose levels. Certain kinds of flavonoids or their glycosides stimulate cells to improve glucose uptake and lower blood glucose levels. We synthesized kaempferol 3-O-neohesperidoside (1), a naturally occurring substance present in Cyathea phalerata Mart., reported to mimic the action of insulin. Synthetic 1 promoted glucose uptake in the cultured cell line, L6. Further studies to determine the core structure responsible for this activity using synthetic compounds revealed Neohesperidose to be the primary pharmacophore. These findings support the use of certain saccharides as a potential novel treatment for diabetes mellitus by replacing or supporting insulin.
Antioxidant flavonoid glycosides from the leaves of Ficus pumila L.[Pubmed:26003366]
Food Chem. 2008 Jul 15;109(2):415-20.
The Okinawan folks in Japan use Ficus pumila L. as a beverage or herbal medicine to treat diabetes and high blood pressure. Four flavonoid glycosides were isolated and identified as rutin (1 and 3), apigenin 6-Neohesperidose (2), kaempferol 3-robinobioside (4) and kaempferol 3-rutinoside (5). Among these compounds, rutin exhibited the strongest antioxidant activity in DPPH radical scavenging assay and superoxide radical inhibition assay. The preparation of Ooitabi leaves in water provide sufficient amount of flavonoid glycosides to the Okinawan although 50% of aqueous ethanol extracted these flavonoid glycosides more effectively. These results show the potential of Ooitabi leaves as a natural source of antioxidant for health management.
Silver complexation and tandem mass spectrometry for differentiation of isomeric flavonoid diglycosides.[Pubmed:15762583]
Anal Chem. 2005 Mar 15;77(6):1761-70.
For detection and differentiation of isomeric flavonoids, electrospray ionization mass spectrometry is used to generate silver complexes of the type (Ag + flavonoid)+. Collisionally activated dissociation (CAD) of the resulting 1:1 silver/flavonoid complexes allows isomer differentiation of flavonoids. Eighteen flavonoid diglycosides constituting seven isomeric series are distinguishable from each other based on the CAD patterns of their silver complexes. Characteristic dissociation pathways allow identification of the site of glycosylation, the type of disaccharide (rutinose versus Neohesperidose), and the type of aglycon (flavonol versus flavone versus flavanone). This silver complexation method is more universal than previous metal complexation methods, as intense silver complexes are observed even for flavonoids that lack the typical metal chelation sites. To demonstrate the feasibility of using silver complexation and tandem mass spectrometry to characterize flavonoids in complex mixtures, flavonoids extracted from grapefruit juice are separated by high-performance liquid chromatography and analyzed via a postcolumn complexation ESI-MS/MS strategy. Diagnostic fragmentation pathways of the silver complexes of the individual eluting flavonoids allow successful identification of the six flavonoids in the extract.
Threshold dissociation and molecular modeling of transition metal complexes of flavonoids.[Pubmed:15694764]
J Am Soc Mass Spectrom. 2005 Feb;16(2):139-51.
The relative threshold dissociation energies of a series of flavonoid/transition metal/auxiliary ligand complexes of the type [MII (flavonoid - H) auxiliary ligand]+ formed by electrospray ionization (ESI) were measured by energy-variable collisionally activated dissociation (CAD) in a quadrupole ion trap (QIT). For each of the isomeric flavonoid diglycoside pairs, the rutinoside (with a 1-6 inter-saccharide linkage) requires a greater CAD energy and thus has a higher dissociation threshold than its neohesperidoside (with a 1-2 inter-saccharide linkage) isomer. Likewise, the threshold energies of complexes containing flavones are higher than those containing flavanones. The monoglycoside isomers also have characteristic threshold energies. The flavonoids that are glycosylated at the 3-O- position tend to have lower threshold energies than those glycosylated at the 7-O- or 4'-O- position, and those that are C- bonded have lower threshold energies than the O- bonded isomers. The structural features that substantially influence the threshold energies include the aglycon type (flavanone versus flavone), the type of disaccharide (rutinose versus Neohesperidose), and the linkage type (O- bonded versus C- bonded). Various computational means were applied to probe the structures and conformations of the complexes and to rationalize the differences in threshold energies of isomeric flavonoids. The most favorable coordination geometry of the complexes has a plane-angle of about 62 degrees , which means that the deprotonated flavonoid and 2,2'-bipyridine within a complex do not reside on the same plane. Stable conformations of five cobalt complexes and five deprotonated flavonoids were identified. The conformations were combined with the point charges and helium accessible surface areas to explain qualitatively the differences in threshold energies for isomeric flavonoids.
Characterization of flavonoids by aluminum complexation and collisionally activated dissociation.[Pubmed:15674859]
J Mass Spectrom. 2005 Mar;40(3):350-63.
Aluminum complexes of the type [Al(III) (flavonoid-H)2]+ are generated by electrospray ionization in order to allow differentiation of isomeric flavonoids by tandem mass spectrometry. The dominant species observed from the aluminum complexation reaction has a 1:2 aluminum(III):flavonoid stoichiometry. Differentiation of 18 flavonoids constituting seven isomeric series was achieved based on the collisionally activated dissociation patterns of the aluminum complexes. Characteristic fragmentation pathways allow identification of the site of glycosylation, the type of saccharide (rutinose versus Neohesperidose) and the type of bond between the C-2 and C-3 atoms (thus distinguishing flavanones from flavonols and flavones). Two stable coordination geometries of the aluminum complex of apigenin were identified. The non-planar structure with a plane-angle of nearly 90 degrees is 25.3 kcal mol-1 more favorable than the planar structure. The conformations of the complexes, which involve multiple interactions between the aglycone and disaccharide portions of the flavonoid with the metal ion, are significantly different for the isomeric flavonoids.


