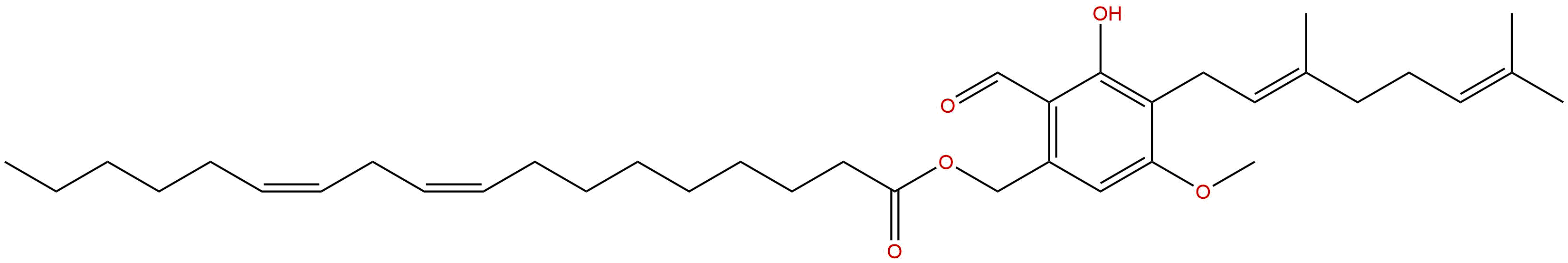
Hericene DCAS# 1343477-87-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1343477-87-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C37H56O5 | M.Wt | 580.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hericene D Dilution Calculator

Hericene D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7216 mL | 8.6081 mL | 17.2161 mL | 34.4323 mL | 43.0404 mL |
| 5 mM | 0.3443 mL | 1.7216 mL | 3.4432 mL | 6.8865 mL | 8.6081 mL |
| 10 mM | 0.1722 mL | 0.8608 mL | 1.7216 mL | 3.4432 mL | 4.304 mL |
| 50 mM | 0.0344 mL | 0.1722 mL | 0.3443 mL | 0.6886 mL | 0.8608 mL |
| 100 mM | 0.0172 mL | 0.0861 mL | 0.1722 mL | 0.3443 mL | 0.4304 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Maltononaose
Catalog No.:BCX0995
CAS No.:6471-60-9
- Maltodecaose
Catalog No.:BCX0994
CAS No.:6082-21-9
- 1,3-Disinapoylglucose
Catalog No.:BCX0993
CAS No.:1423128-15-7
- S-Isogambogic acid
Catalog No.:BCX0992
CAS No.:942623-57-6
- Forbesione
Catalog No.:BCX0991
CAS No.:180961-63-1
- (9Z,11E)-13-Oxo-9,11-octadecadienoic Acid
Catalog No.:BCX0990
CAS No.:54739-30-9
- 5-Methoxyisosakuranin
Catalog No.:BCX0989
CAS No.:59942-61-9
- Violaxanthin
Catalog No.:BCX0988
CAS No.:126-29-4
- Antheraxanthin
Catalog No.:BCX0987
CAS No.:640-03-9
- Notoginsenoside R3
Catalog No.:BCX0986
CAS No.:87741-76-2
- 1,2-Disinapoylglucose
Catalog No.:BCX0985
CAS No.:91095-79-3
- Caffeic acid 4-O-glucopyranoside
Catalog No.:BCX0984
CAS No.:17093-82-2
- Neopuerarin B
Catalog No.:BCX0997
CAS No.:1150314-39-8
- Homodihydrocapsaicin II
Catalog No.:BCX0998
CAS No.:71239-21-9
- Apigenin triacetate
Catalog No.:BCX0999
CAS No.:3316-46-9
- Anisomelic acid
Catalog No.:BCX1000
CAS No.:59632-76-7
- Ovatodiolide
Catalog No.:BCX1001
CAS No.:3484-37-5
- Neohesperidose
Catalog No.:BCX1002
CAS No.:17074-02-1
- α-Glucosyl Hesperidin
Catalog No.:BCX1003
CAS No.:161713-86-6
- Hericene B
Catalog No.:BCX1004
CAS No.:157207-55-1
- Hericene A
Catalog No.:BCX1005
CAS No.:157207-54-0
- Euphorbetin
Catalog No.:BCX1006
CAS No.:35897-99-5
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities.[Pubmed:35408555]
Molecules. 2022 Mar 27;27(7):2157.
Hericium erinaceus, a culinary and medicinal mushroom, is widely consumed in Asian countries. Chemical investigation on the fruiting bodies of Hericium erinaceus led to the isolation of one new ergostane-type sterol fatty acid ester, erinarol K (1); and eleven known compounds: 5alpha,8alpha -epidioxyergosta-6,22-dien-3beta-yl linoleate (2); ethyl linoleate (3); linoleic acid (4); hericene A (5); Hericene D (6); hericene E (7); ergosta-4,6,8(14),22-tetraen-3-one (8); hericenone F (9); ergosterol (10); ergosterol peroxide (11); 3beta,5alpha,6alpha,22E-ergosta-7,22-diene-3,5,6-triol 6-oleate (12). The chemical structures of the compounds were determined by 1D and 2D NMR (nuclear magnetic resonance) spectroscopy, mass spectra, etc. Anti-inflammatory effects of the isolated aromatic compounds (5-7, 9) were evaluated in terms of inhibition of pro-inflammatory mediator (TNF-alpha, IL-6 and NO) production in lipopolysaccharide (LPS)-stimulated murine RAW 264.7 macrophage cells. The results showed that compounds 5 and 9 exhibited moderate activity against TNF-alpha (IC(50): 78.50 muM and 62.46 muM), IL-6 (IC(50): 56.33 muM and 48.50 muM) and NO (IC(50): 87.31 muM and 76.16 muM) secretion. These results supply new information about the secondary metabolites of Hericium erinaceus and their anti-inflammatory effects.
Total Synthesis, Structure Revision, and Neuroprotective Effect of Hericenones C-H and Their Derivatives.[Pubmed:33492133]
J Org Chem. 2021 Feb 5;86(3):2602-2620.
The first total syntheses of hericenones C-H and "putative 3-hydroxyhericenone F" were achieved. Highlights of the synthesis include the straightforward construction of the resorcinol core and geranyl side chain, assembly of the natural product skeleton by sequential O-geranylation and a clay/zeolite-mediated O --> C rearrangement reaction, and a biomimetic cyclization to form a variety of bicyclic natural hericenones and their congeners. The structure of the "putative 3-hydroxyhericenone F" was revised as the 5-exo cyclization product (named: hericenone Z) of epoxyhericenone C through in-depth analyses of the cyclization modes in addition to NMR spectroscopic studies. To gain insights into the biological functions of geranyl-resorcinols in Hericium erinaceus, potential neuroprotective effects against endoplasmic reticulum (ER) stress-dependent cell death were evaluated systematically to clarify a fundamental structure-activity relationship. Among the compounds assayed, the linoleate-containing hericenone analogue, i.e., the regioisomer of Hericene D, was found to possess the most potent neuroprotective effect against tunicamycin and thapsigargin-induced ER stress-dependent cell death.
Characterization of alpha-glucosidase inhibitory constituents of the fruiting body of lion's mane mushroom (Hericium erinaceus).[Pubmed:32738392]
J Ethnopharmacol. 2020 Nov 15;262:113197.
ETHNOPHARMACOLOGICAL RELEVANCE: Hericium erinaceus, commonly called lion's mane mushroom, is an edible and medicinal mushroom that has been traditionally used for the treatment of metabolic disorders, gastrointestinal diseases and memory impairment. In this study, potential anti-hyperglycemic constituents were identified to support the traditional usage of H. erinaceus. MATERIALS AND METHODS: The components of H. erinaceus were purified using various column chromatography techniques. The structure of the separated compounds was determined based on spectroscopic data analysis, i.e., 1D and 2D NMR analysis. The anti-hyperglycemic activity of the isolated compounds was evaluated by measuring the inhibitory effects on alpha-glucosidase activity. Molecular docking analysis was also conducted for elucidation of alpha-glucosidase inhibitory activity of isolated compounds. RESULTS: Ten compounds including four new compounds, erinacenols A-D (1-4), were isolated from the fruiting bodies of H. erinaceus. Investigation of the anti-hyperglycemic effect of isolated compounds demonstrated that erinacenol D (4), 4-[3',7'-dimethyl-2',6'-octadienyl]-2-formyl-3-hydroxy-5-methyoxybenzylalcohol (6), hericene A (7), Hericene D (8) and hericenone D (9) strongly inhibited alpha-glucosidase activity with IC(50) values of <20 muM. The structure activity relationship suggested the importance of long side chain for alpha-glucosidase inhibitory activity. Further analysis by molecular docking demonstrated the interaction of alpha-glucosidase and isolated compounds, which supported the inhibitory activity of alpha-glucosidase. CONCLUSION: Our present study demonstrated the beneficial effect of H. erinaceus by characterization of alpha-glucosidase inhibitory compounds, including four new compounds. This approach can be valuable support for the traditional use of H. erinaceus for the treatment of diabetes and metabolic diseases, which needs to be clarified by further in-vivo study.


