Anisomelic acidCAS# 59632-76-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59632-76-7 | SDF | Download SDF |
| PubChem ID | 90473786.0 | Appearance | Powder |
| Formula | C20H26O4 | M.Wt | 330.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
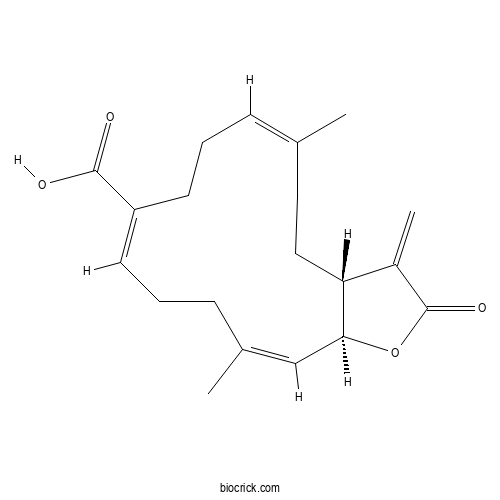
| Chemical Name | (3aR,6Z,10E,14Z,15aS)-6,14-dimethyl-3-methylidene-2-oxo-3a,4,5,8,9,12,13,15a-octahydrocyclotetradeca[b]furan-10-carboxylic acid | ||
| SMILES | CC1=CCCC(=CCCC(=CC2C(CC1)C(=C)C(=O)O2)C)C(=O)O | ||
| Standard InChIKey | SORYERHBQFTRIK-MDLRLGSJSA-N | ||
| Standard InChI | InChI=1S/C20H26O4/c1-13-6-4-8-16(19(21)22)9-5-7-14(2)12-18-17(11-10-13)15(3)20(23)24-18/h6,9,12,17-18H,3-5,7-8,10-11H2,1-2H3,(H,21,22)/b13-6-,14-12-,16-9+/t17-,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Anisomelic acid Dilution Calculator

Anisomelic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0265 mL | 15.1323 mL | 30.2645 mL | 60.529 mL | 75.6613 mL |
| 5 mM | 0.6053 mL | 3.0265 mL | 6.0529 mL | 12.1058 mL | 15.1323 mL |
| 10 mM | 0.3026 mL | 1.5132 mL | 3.0265 mL | 6.0529 mL | 7.5661 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6053 mL | 1.2106 mL | 1.5132 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6053 mL | 0.7566 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Apigenin triacetate
Catalog No.:BCX0999
CAS No.:3316-46-9
- Homodihydrocapsaicin II
Catalog No.:BCX0998
CAS No.:71239-21-9
- Neopuerarin B
Catalog No.:BCX0997
CAS No.:1150314-39-8
- Hericene D
Catalog No.:BCX0996
CAS No.:1343477-87-1
- Maltononaose
Catalog No.:BCX0995
CAS No.:6471-60-9
- Maltodecaose
Catalog No.:BCX0994
CAS No.:6082-21-9
- 1,3-Disinapoylglucose
Catalog No.:BCX0993
CAS No.:1423128-15-7
- S-Isogambogic acid
Catalog No.:BCX0992
CAS No.:942623-57-6
- Forbesione
Catalog No.:BCX0991
CAS No.:180961-63-1
- (9Z,11E)-13-Oxo-9,11-octadecadienoic Acid
Catalog No.:BCX0990
CAS No.:54739-30-9
- 5-Methoxyisosakuranin
Catalog No.:BCX0989
CAS No.:59942-61-9
- Violaxanthin
Catalog No.:BCX0988
CAS No.:126-29-4
- Ovatodiolide
Catalog No.:BCX1001
CAS No.:3484-37-5
- Neohesperidose
Catalog No.:BCX1002
CAS No.:17074-02-1
- α-Glucosyl Hesperidin
Catalog No.:BCX1003
CAS No.:161713-86-6
- Hericene B
Catalog No.:BCX1004
CAS No.:157207-55-1
- Hericene A
Catalog No.:BCX1005
CAS No.:157207-54-0
- Euphorbetin
Catalog No.:BCX1006
CAS No.:35897-99-5
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
- Isomaltoheptaose
Catalog No.:BCX1009
CAS No.:6513-12-8
- Isomaltohexaose
Catalog No.:BCX1010
CAS No.:6175-02-6
- Isomaltopentaose
Catalog No.:BCX1011
CAS No.:6082-32-2
- Isomaltotetraose
Catalog No.:BCX1012
CAS No.:35997-20-7
Identification and semisynthesis of (-)-anisomelic acid as oral agent against SARS-CoV-2 in mice.[Pubmed:36601138]
Natl Sci Rev. 2022 Aug 26;9(11):nwac176.
(-)-Anisomelic acid, isolated from Anisomeles indica (L.) Kuntze (Labiatae) leaves, is a macrocyclic cembranolide with a trans-fused alpha-methylene-gamma-lactone motif. Anisomelic acid effectively inhibits SARS-CoV-2 replication and viral-induced cytopathic effects with an EC(50) of 1.1 and 4.3 muM, respectively. Challenge studies of SARS-CoV-2-infected K18-hACE2 mice showed that oral administration of Anisomelic acid and subcutaneous dosing of remdesivir can both reduce the viral titers in the lung tissue at the same level. To facilitate drug discovery, we used a semisynthetic approach to shorten the project timelines. The enantioselective semisynthesis of Anisomelic acid from the naturally enriched and commercially available starting material (+)-costunolide was achieved in five steps with a 27% overall yield. The developed chemistry provides opportunities for developing anisomelic-acid-based novel ligands for selectively targeting proteins involved in viral infections.
Synthesis and Evaluation of Anisomelic acid-like Compounds for the Treatment of HPV-Mediated Carcinomas.[Pubmed:31889069]
Sci Rep. 2019 Dec 30;9(1):20295.
The vast majority of cervical and 75% of oropharyngeal carcinomas are triggered by infection with a type of high-risk oncogenic human papillomavirus (HPV). It is well-known that E6 and E7 oncoproteins are critical for viral-induced cancer, and hence, they represent valuable targets for therapeutic intervention in HPV-mediated cancers. Our earlier research on the cembranoid, Anisomelic acid (AA) showed that, AA has the potential to induce apoptosis in HPV cells by the depletion of E6 and E7 oncoproteins. The present study describes the structure-activity relationship and the evaluation of synthetic AA like compounds, i.e simplified cembranoid-like structures, as HPV inhibitors against some papilloma cell lines. Both from experimental and computational results, we observed that these compounds induced apoptosis by the same E6/E7-based mechanism as AA, but at earlier time points, thus being far more effective than AA. Further, the data indicated that only part of the structure of AA is required for the molecular action. Based on these results, we identified some novel and potential compounds for specific treatment of HPV-associated carcinomas.
Plasma Protein Binding of Anisomelic Acid: Spectroscopy and Molecular Dynamic Simulations.[Pubmed:28024399]
J Chem Inf Model. 2016 Dec 27;56(12):2401-2412.
Anisomelic acid (AA) is a macrocyclic cembranolide compound extracted from Anisomeles herbal species. Recently, we have shown that AA possesses both anticancer and antiviral activity. However, to date, the plasma protein binding properties of AA are unknown. Here, we describe the molecular interactions of AA with two serum proteins, human serum albumin (HSA) and bovine serum albumin (BSA), adopting multiple physicochemical methods. Besides, molecular docking and dynamics simulations were performed to predict the interaction mode and the dynamic behavior of AA with HSA and BSA. The experimental results revealed that hydrophobic forces play a significant part in the interaction of AA to HSA and BSA. The outcomes of the principal components analysis (PCA) of the poses based on root-mean-squared distances showed less variation in AA-HSA, opposed to what is seen for BSA-AA. Furthermore, binding free energies estimated for AA-HSA and AA-BSA complexes at different temperatures (298, 303, 308, and 313 K) based on molecular mechanics-generalized Born surface area (MMGBSA) approaches were well correlated with our experimental results.
Targeted delivery of a novel anticancer compound anisomelic acid using chitosan-coated porous silica nanorods for enhancing the apoptotic effect.[Pubmed:26214194]
Biomater Sci. 2015 Jan;3(1):103-11.
Targeted cancer therapies are currently a strong focus in biomedical research. The most common approach is to use nanocarrier-based targeting to specifically deliver conventional anticancer drugs to enhance their therapeutic efficacy, increase bioavailability, and decrease the side-effects on normal cells. A step further towards higher specificity and efficacy would be to employ specific novel drugs along with specific nanocarrier-based targeting. Our recent studies have demonstrated that a plant-derived diterpenoid compound, Anisomelic acid (AA), induces apoptosis in cervical cancer cells. In this work, we describe the development of a folic acid (FA)-targeted AA delivery system using chitosan-coated rod-shaped mesoporous silica particles (Chitosan-NR-MSP). The cellular internalization and uptake enhancement of the FA-Chitosan-NR-MSP towards cancerous folate receptor (FR)-positive (SiHa and HeLa) and/or normal FR-negative (HEK 293) cells were assessed, which indicated that the intracellular uptake of FA-conjugated Chitosan-NR-MSP was more target-specific. Furthermore, the induction of apoptosis by AA-loaded chitosan-coated rod-shaped particles on SiHa cells was studied. By employing caspase-3 activation and PARP cleavage as measure of apoptosis, the FA-particle mediated AA treatment was clearly more effective, significantly enhancing apoptosis in comparison to non-targeted Chitosan-NR-MSP or free AA in SiHa cells, suggesting that the FA-Chitosan-NR-MSPs can be potentially utilized as a drug delivery system for cervical cancer treatment.
Novel action modality of the diterpenoid anisomelic acid causes depletion of E6 and E7 viral oncoproteins in HPV-transformed cervical carcinoma cells.[Pubmed:24565908]
Biochem Pharmacol. 2014 May 15;89(2):171-84.
Cervical cancer, the second most common malignancy among women, is mainly caused by human papilloma virus (HPV) infection. In HPV-positive cervical cancer cells, the activity of p53 and the induction of p21 are inhibited by the HPV oncoproteins E6 and E7. Therefore, blocking the activity of E6 and E7 would serve as an important therapeutic target in these cancer cells. In this study, Anisomelic acid (AA), a natural compound belonging to the same diterpenoid family of bioactive compounds as taxol, was found to deplete the E6 and E7 proteins in HPV-positive cervical cancer cells. Consequently, p53 and the p53-responsive gene, p21, were dramatically induced, leading to G2/M-phase cell cycle arrest. AA-mediated cell cycle arrest and p21 expression were canceled when p53 was down-regulated by p53-shRNA. AA also induced p53-independent intrinsic apoptosis by depletion of the cellular inhibitor of apoptosis protein 2 (cIAP2) whose proteosomal degradation is inhibited by E6. The in ovo chick embryo chorioallantoic membrane (CAM) assay showed that Anisomelic acid inhibited the tumor growth of the cervical cancer SiHa cells. AA is revealed to hold a novel action modality based on specific targeting of the HPV oncoproteins, which restores p53-mediated growth arrest and induces apoptosis by terminating E6-mediated cIAP2 stabilization.
Analysis of the Cytotoxic Potential of Anisomelic Acid Isolated from Anisomeles malabarica.[Pubmed:23833721]
Sci Pharm. 2013 Apr-Jun;81(2):559-66.
Anisomelic acid (AA), one of the major compounds in Anisomeles malabarica, was tested for its cytotoxicity and apoptosis-inducing potential in breast and cervical cancer cells. The MTT assay for cell viability indicated that AA is cytotoxic to all of the four cell lines tested in a dose- and duration-dependent manner. Acridine Orange & Ethidium Bromide (AO & EB) and Hoechst 33258 staining of AA-treated cells revealed typical apoptotic morphology such as condensed chromatin and formation of apoptotic bodies. The comet assay revealed DNA strand break(s), indicating that AA induces DNA damage which culminates in apoptosis. Thus, the study revealed the anti-proliferative and apoptosis-inducing properties of AA in both breast and cervical cancer cells. Therefore, Anisomelic acid offers potential for application in breast and cervical cancer therapy.
Biological active macrocyclic diterpenoids from chinese drug "Fang Feng Cao"; II. Derivatives of ovatodiolids and their cytotoxity.[Pubmed:17345312]
Planta Med. 1986 Aug;(4):297-9.
Isolated macrocyclic diterpenoids, ovatodiolide ( 1), 4, 5-epoxyovatodiolide ( 2), Anisomelic acid ( 3), 4, 7-oxycycloAnisomelic acid ( 4), and 4-methylene-5-oxoAnisomelic acid ( 6), from the Chinese crude drug "Fang Feng Caordquo;, and the derivatives of the isolated compounds were tested for their growth inhibiting activities in culture of KB cell line. Besides, the effects of 1 on Ehrlich carcinoma in mice are reported.


