GlucodigifucosideCAS# 2446-63-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2446-63-1 | SDF | Download SDF |
| PubChem ID | 164965 | Appearance | Powder |
| Formula | C35H54O13 | M.Wt | 682.8 |
| Type of Compound | Cardenolides and its Sapogenins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
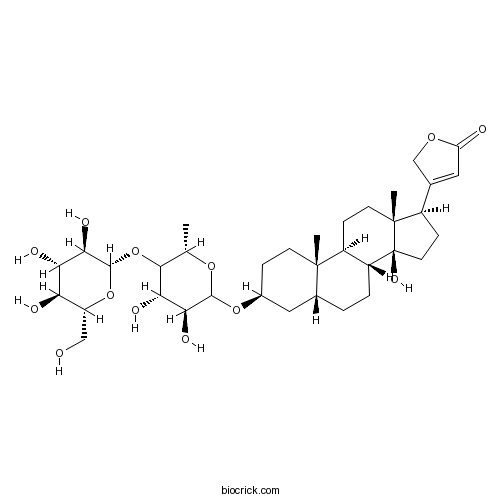
| Chemical Name | 3-[(3S,5R,8R,9S,10S,13R,14S,17R)-3-[(3S,4S,6S)-3,4-dihydroxy-6-methyl-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-14-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | ||
| SMILES | CC1C(C(C(C(O1)OC2CCC3(C(C2)CCC4C3CCC5(C4(CCC5C6=CC(=O)OC6)O)C)C)O)O)OC7C(C(C(C(O7)CO)O)O)O | ||
| Standard InChIKey | OQZGLOBKVNEEPK-HYYJYYHTSA-N | ||
| Standard InChI | InChI=1S/C35H54O13/c1-16-30(48-32-28(41)26(39)25(38)23(14-36)47-32)27(40)29(42)31(45-16)46-19-6-9-33(2)18(13-19)4-5-22-21(33)7-10-34(3)20(8-11-35(22,34)43)17-12-24(37)44-15-17/h12,16,18-23,25-32,36,38-43H,4-11,13-15H2,1-3H3/t16-,18+,19-,20+,21-,22+,23+,25+,26-,27-,28+,29-,30?,31?,32-,33-,34+,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucodigifucoside Dilution Calculator

Glucodigifucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4646 mL | 7.3228 mL | 14.6456 mL | 29.2912 mL | 36.6139 mL |
| 5 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL | 5.8582 mL | 7.3228 mL |
| 10 mM | 0.1465 mL | 0.7323 mL | 1.4646 mL | 2.9291 mL | 3.6614 mL |
| 50 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| 100 mM | 0.0146 mL | 0.0732 mL | 0.1465 mL | 0.2929 mL | 0.3661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trimethyl phosphate
Catalog No.:BCN9059
CAS No.:512-56-1
- N-Phenethylbenzamide
Catalog No.:BCN9058
CAS No.:3278-14-6
- Warfarin sodium
Catalog No.:BCN9057
CAS No.:129-06-6
- Flavokawain A
Catalog No.:BCN9056
CAS No.:37951-13-6
- 5'-Guanylic acid
Catalog No.:BCN9055
CAS No.:85-32-5
- Xanthosine
Catalog No.:BCN9054
CAS No.:146-80-5
- 5'-Cytidylic acid
Catalog No.:BCN9053
CAS No.:63-37-6
- DL-Tartaric acid
Catalog No.:BCN9052
CAS No.:133-37-9
- Mirificin-4'-O-glucoside
Catalog No.:BCN9051
CAS No.:168035-01-6
- Bisisorhapontigenin D
Catalog No.:BCN9050
CAS No.:
- Hydroquinone
Catalog No.:BCN9049
CAS No.:123-31-9
- 1αH,5αH-guaia-6-ene-4β,10β-diol
Catalog No.:BCN9048
CAS No.:2013537-81-8
- (±)-Naringenin
Catalog No.:BCN9061
CAS No.:67604-48-2
- α-L-Rhamnopyranose
Catalog No.:BCN9062
CAS No.:6014-42-2
- D-Ribose
Catalog No.:BCN9063
CAS No.:50-69-1
- Alizarin 1-methyl ether
Catalog No.:BCN9064
CAS No.: 6170-06-5
- Eugenol acetate
Catalog No.:BCN9065
CAS No.:93-28-7
- Citral
Catalog No.:BCN9066
CAS No.:5392-40-5
- 10-hydroxydec-2-enoic acid
Catalog No.:BCN9067
CAS No.:14113-05-4
- Citronellal
Catalog No.:BCN9068
CAS No.:106-23-0
- Benzyl alcohol
Catalog No.:BCN9069
CAS No.:100-51-6
- (-)-Menthone
Catalog No.:BCN9070
CAS No.:14073-97-3
- (+)-Fenchone
Catalog No.:BCN9071
CAS No.:4695-62-9
- Alphalipoic acid
Catalog No.:BCN9072
CAS No.:1077-28-7
Profiling and structural analysis of cardenolides in two species of Digitalis using liquid chromatography coupled with high-resolution mass spectrometry.[Pubmed:32035664]
J Chromatogr A. 2020 May 10;1618:460903.
Plants of the Digitalis genus contain a cocktail of cardenolides commonly prescribed to treat heart failure. Cardenolides in Digitalis extracts have been conventionally quantified by high-performance liquid chromatography yet the lack of structural information compounded with possible co-eluents renders this method insufficient for analyzing cardenolides in plants. The goal of this work is to structurally characterize cardiac glycosides in fresh-leaf extracts using liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) that provides measured accurate mass. Fragmentation of cardenolides is featured by sequential loss of sugar units while the steroid aglycone moieties undergo stepwise elimination of hydroxyl groups, which distinguishes different aglycones. Using a reverse-phase LC column, the sequence of elution follows: diginatigenin-->digoxigenin-->gitoxigenin-->gitaloxigenin-->digitoxigenin for cardenolides with the same sugar units but different aglycones. A linear range of 0.8-500 ng ml(-1) has been achieved for digoxigenin, beta-acetyldigoxin, and digitoxigenin with limits of detection ranging from 0.09 to 0.45 ngml(-1). A total of seventeen cardenolides have been detected with lanatoside A, C, and E as major cardenolides in Digitalis lanata while seven have been found in Digitalis purpurea including purpurea glycoside A, B, and E. Surprisingly, Glucodigifucoside in D. lanata and verodoxin and digitoxigenin fucoside in D. purpurea have also been found as major cardenolides. As the first MS/MS-based method developed for analyzing cardenolides in plant extracts, this method serves as a foundation for complete identification and accurate quantification of cardiac glycosides, a necessary step towards understanding the biosynthesis of cardenolide in plants.
Cardenolide glycosides from the seeds of Digitalis purpurea exhibit carcinoma-specific cytotoxicity toward renal adenocarcinoma and hepatocellular carcinoma cells.[Pubmed:25345317]
Biosci Biotechnol Biochem. 2015;79(2):177-84.
Four cardenolide glycosides, Glucodigifucoside (2), 3'-O-acetylglucoevatromonoside (9), digitoxigenin 3-O-beta-D-glucopyranosyl-(1 --> 4)-beta-D-glucopyranosyl-(1 --> 4)-3-O-acetyl-beta-D-digitoxopyranoside (11), and purpureaglycoside A (12), isolated from the seeds of Digitalis purpurea, exhibited potent cytotoxicity against human renal adenocarcinoma cell line ACHN. These compounds exhibited significantly lower IC50 values against ACHN than that against normal human renal proximal tubule-derived cell line HK-2. In particular, 2 exhibited the most potent and carcinoma-specific cytotoxicity, with a sixfold lower IC50 value against ACHN than that against HK-2. Measurement of cyclin-dependent kinase inhibitor levels revealed that upregulation of p21/Cip1 expression was involved in the carcinoma-specific cytotoxicity of 2. Further, compound 2 also exhibited the carcinoma-specific cytotoxicity toward hepatocellular carcinoma cell line.
Cardiac glycosides in partly submerged shoots of Digitalis lanata.[Pubmed:8302954]
Planta Med. 1993 Dec;59(6):539-45.
Shoot cultures were established from axillary buds (11 strains) or seeds (1 strain) of individual Digitalis lanata Ehrh. plants and propagated partially submerged in liquid medium. Five of these shoot culture strains were characterized with regard to their growth and cardenolide content. The cultures were observed for more than one year and found to be relatively stable with regard to their growth and cardenolide spectrum and yield. The strains examined differed in terms of their total cardenolide yield, which ranged from about 30 nmol g DW-1 to almost 1000 nmol g DW-1. Cardenolide content was correlated with leaf size and development. Depending on the strain investigated up to ten different cardenolides could be detected by HPLC. The main cardenolides were identified by comparing HPLC and TLC results with those of authentic samples and chemical degradation as being the mono- and diglycosides Glucodigifucoside, glucoverodoxin, odorobioside G, and odoroside H; minor amounts of digitalinum verum and glucoevatromonoside were also found. In addition, the tetrasaccharides lanatoside A and C were present. The shoots were cardenolide-free when cultivated in the dark for more than 30 weeks, but regained their characteristic cardenolide profile when transferred back to light. For the dark cultivation of chlorophyll-free cultures a medium containing 3.5% glucose was found to be optimal.
Cardenolides fromErysimum cheiranthoides: Feeding deterrents toPieris rapae larvae.[Pubmed:24249167]
J Chem Ecol. 1993 Jul;19(7):1355-69.
Larvae of the cabbage butterfly,Pieris rapae, refuse to feed on the wild mustard,Erysimum cheiranthoides, due to the presence of alcoholextractable deterrents. The active components were extracted inton-BuOH, and this extract was separated into four fractions (I-IV) by reverse-phase HPLC. Fractions III and IV retained the feeding deterrent activity. The activity of fraction III was found to be due to the cardenolide diglycosides 1 and 2, which were previously reported as oviposition deterrents for gravidP. rapae butterflies. Three active compounds were isolated from fraction IV by column chromatography on silica gel followed by reverse-phase HPLC. These compounds were identified as a monoglycoside, digitoxigenin 3-O-beta-D-glucoside (4), and two diglycosides, glucodigigulomethyloside (5) and Glucodigifucoside (6). An additional cardenolide isolated from fraction II was identified as cheirotoxin (7). All compounds were identified by UV, NMR ((1)H and(13)C), and mass spectrometry, as well as hydrolysis experiments. The feeding deterrent activity of these compounds was compared with that of related commercially available chemicals and other compounds isolated fromE. cheiranthoides.
Separation of cardiac glycosides by micellar electrokinetic capillary electrophoresis.[Pubmed:8486748]
J Chromatogr. 1993 Apr 16;635(2):319-27.
The separation of mixtures of primary and secondary cardiac glycosides by micellar electrokinetic capillary electrophoresis modified by cyclodextrins, urea and sodium cholate proved to be suitable for the determination of these hydrophobic compounds. It was possible to distinguish the two anomeric cardenolides Glucodigifucoside and glucodigiglucomethyloside with all three buffer systems. Electropherograms of crude plant cell extracts from Digitalis lanata were obtained with this method.
Cardiac Glycosides from the Leaves of Digitalis cariensis.[Pubmed:17268958]
Planta Med. 1987 Feb;53(1):43-6.
From the leaves of DIGITALIS CARIENSIS in addition to the known glycosides, three new cardenolides, a major and two minor ones, have been isolated and their structures established as gluco-gitoxigenine-glucomethyloside (gitoxigenine-3- O-beta- D-glucosyl-beta- D-glucomethyloside), gitoxigenine-3- O-beta- D-glucosyl-beta- D-glucomethylosyl-beta- D-bisdigitoxoside, and gitoxigenine-3- O-beta- D-glucosyl-beta- D-glucomethylosyl-beta- D-digitoxide, respectively, by chemical evidence and FAB-MS. Except for the unstable glucolanadoxine, the known major components isolated from the leaves are glucogitoroside, glucoevatromonoside, digitalinum verum, gluco-digitoxigenine-glucomethyloside, Glucodigifucoside, and glucogitofucoside. A total of 23 cardenolides present in the leaves in trace amounts were also identified.


