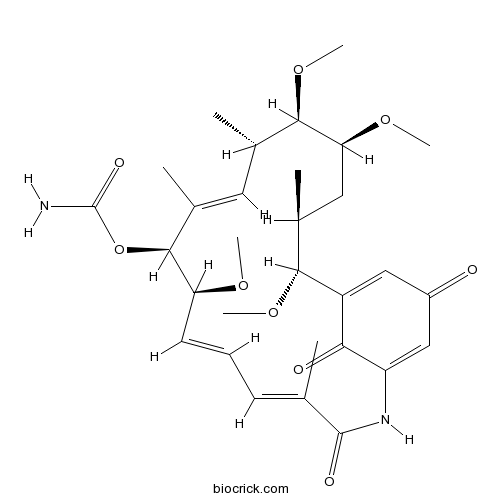
Herbimycin Aantibiotic and inhibitor of non-receptor tyrosine kinases CAS# 70563-58-5 |
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70563-58-5 | SDF | Download SDF |
| PubChem ID | 6436247 | Appearance | Powder |
| Formula | C30H42N2O9 | M.Wt | 574.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | [(4E,6E,8S,9S,10E,12S,13R,14S,16S,17R)-8,13,14,17-tetramethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl] carbamate | ||
| SMILES | CC1CC(C(C(C=C(C(C(C=CC=C(C(=O)NC2=CC(=O)C=C(C1OC)C2=O)C)OC)OC(=O)N)C)C)OC)OC | ||
| Standard InChIKey | MCAHMSDENAOJFZ-GDYSMBPZSA-N | ||
| Standard InChI | InChI=1S/C30H42N2O9/c1-16-10-9-11-23(37-5)28(41-30(31)36)18(3)12-17(2)27(40-8)24(38-6)13-19(4)26(39-7)21-14-20(33)15-22(25(21)34)32-29(16)35/h9-12,14-15,17,19,23-24,26-28H,13H2,1-8H3,(H2,31,36)(H,32,35)/b11-9+,16-10+,18-12+/t17-,19-,23-,24-,26+,27+,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ansamycin antibiotic that acts as a Src family kinase inhibitor. Binds to the SH domain and inhibits the activity of p60v-src and p210BCR-ABL. Exhibits antiangiogenic activity in endothelial cells in vitro. Also inhibits Hsp90 and impairs recovery from heat shock. |

Herbimycin A Dilution Calculator

Herbimycin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7401 mL | 8.7006 mL | 17.4013 mL | 34.8026 mL | 43.5032 mL |
| 5 mM | 0.348 mL | 1.7401 mL | 3.4803 mL | 6.9605 mL | 8.7006 mL |
| 10 mM | 0.174 mL | 0.8701 mL | 1.7401 mL | 3.4803 mL | 4.3503 mL |
| 50 mM | 0.0348 mL | 0.174 mL | 0.348 mL | 0.6961 mL | 0.8701 mL |
| 100 mM | 0.0174 mL | 0.087 mL | 0.174 mL | 0.348 mL | 0.435 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Herbimycin A is an antibiotic and a selective inhibitor of non-receptor tyrosine kinases [1].
Antibiotics are a type of antimicrobial used in the treatment of bacterial infection. Non-receptor tyrosine kinases (nRTKs) are cytoplasmic enzymes and catalyse the transfer of a phosphate group from a nucleoside triphosphate donor to tyrosine residues in proteins.
Herbimycin A is a selective non-receptor tyrosine kinases inhibitor. Herbimycin A bound to the reactive SH domains of p60v-src and p210BCR-ABL and inactivated their activity [1].
In a retinopathy prematurity rat model, Herbimycin A inhibited capillary tube formation and bovine retinal microvascular endothelial cell proliferation induced by vascular endothelial growth factor in a dose-dependent way. Also, Herbimycin A reduced pre-retinal neovascularization by 63% and 41% in oxygen-treated rats and herbimycin-injected eyes, respectively [2]. In rats exposed to heat stress, Herbimycin A exhibited thermotolerance and significantly reduced apoptosis of hepatocytes. Also, Herbimycin A inhibited caspase-3 activation [3].
References:
[1]. Fukazawa H, Uehara Y, Murakami Y, et al. Labeling of v-Src and BCR-ABL tyrosine kinases with [14C]herbimycin A and its use in the elucidation of the kinase inactivation mechanism. FEBS Lett, 1994, 340(3): 155-158.
[2]. McCollum GW, Rajaratnam VS, Bullard LE, et al. Herbimycin A inhibits angiogenic activity in endothelial cells and reduces neovascularization in a rat model of retinopathy of prematurity. Exp Eye Res, 2004, 78(5): 987-995.
[3]. Sachidhanandam SB, Lu J, Low KS, et al. Herbimycin A attenuates apoptosis during heat stress in rats. Eur J Pharmacol, 2003, 474(1): 121-128.
- Daurisoline
Catalog No.:BCN2675
CAS No.:70553-76-3
- Aflatrem
Catalog No.:BCN7414
CAS No.:70553-75-2
- NS 3763
Catalog No.:BCC7275
CAS No.:70553-45-6
- α-Conotoxin PnIA
Catalog No.:BCC5978
CAS No.:705300-84-1
- 3,5-Dimethoxybenzylalcohol
Catalog No.:BCN3760
CAS No.:705-76-0
- 2'-Hydroxy-5'-methoxyacetophenone
Catalog No.:BCN4270
CAS No.:705-15-7
- ARP 100
Catalog No.:BCC2370
CAS No.:704888-90-4
- Mitoxantrone HCl
Catalog No.:BCC4924
CAS No.:70476-82-3
- Petasinoside
Catalog No.:BCN1989
CAS No.:70474-34-9
- Petasinine
Catalog No.:BCN1988
CAS No.:70474-33-8
- Schizanthine A
Catalog No.:BCN1936
CAS No.:70474-24-7
- Corymbol
Catalog No.:BCN6617
CAS No.:7047-54-3
- Yunaconitine
Catalog No.:BCN6261
CAS No.:70578-24-4
- Shizukanolide A
Catalog No.:BCN8021
CAS No.:70578-36-8
- Obtusin
Catalog No.:BCC8223
CAS No.:70588-05-5
- Chrysoobtusin
Catalog No.:BCC8309
CAS No.:70588-06-6
- 19alpha-Hydroxyfern-7-ene
Catalog No.:BCN7405
CAS No.:70588-12-4
- 14beta-Benzoyloxy-2-deacetylbaccatin VI
Catalog No.:BCN1373
CAS No.:705973-69-9
- Phlorizin dihydrate
Catalog No.:BCN2584
CAS No.:7061-54-3
- Boc-D-Tyr-OH
Catalog No.:BCC3463
CAS No.:70642-86-3
- Anisotropine Methylbromide; Octatropine Methylbromide
Catalog No.:BCC8120
CAS No.:70642-90-9
- Z-D-Lys-OH
Catalog No.:BCC2761
CAS No.:70671-54-4
- Catharanthine Sulfate
Catalog No.:BCN3859
CAS No.:70674-90-7
- Canniprene
Catalog No.:BCN4271
CAS No.:70677-47-3
C15-methoxyphenylated 18-deoxy-herbimycin A analogues, their in vitro anticancer activity and heat shock protein 90 binding affinity.[Pubmed:27476419]
Bioorg Med Chem Lett. 2016 Sep 1;26(17):4287-91.
Benzoquinone ansamycins are important leads for the discovery of novel inhibitors of heat shock protein 90 (Hsp90), a promising target of cancer chemotherapeutics. Intrinsic hepatotoxicity caused by the benzoquinone moiety appeared to be a serious limitation to the development of these compounds. To solve this problem by rational structure optimization, a short series of C18-deoxy analogues of Herbimycin A were designed based on putative interactions between the compound and the protein. Chemical synthesis of the target molecules were attempted by following the established synthetic route to the natural product, but resulted in the isolation of four serendipitous C15 phenylated final products. In vitro antiproliferative activity and Hsp90 binding affinity of the compounds were determined, suggesting the C18-oxygen of Herbimycin A is removable and bulky lipophilic groups can be accommodated at C15 without loss of activity.
17-Allylamino-17-demethoxygeldanamycin and Herbimycin A Induce Cell Death by Modulating beta-Catenin and PI3K/AKT Signaling in FRO Anaplastic Thyroid Carcinoma Cells.[Pubmed:26408708]
Anticancer Res. 2015 Oct;35(10):5453-60.
AIM: The aim of the present study was to evaluate the effect of heat-shock protein 90 (HSP90) inhibitors, 17-allylamino-17-demethoxygeldanamycin (17-AAG) and Herbimycin A (HMA) on survival of anaplastic thyroid carcinoma (ATC) cells. MATERIALS AND METHODS: Antitumor activities of 17-AAG and HMA were investigated in FRO ATC cells. RESULTS: In FRO ATC cells, 17-AAG and HMA caused cell death with concomitant changes in the expression of HSP90 client proteins, increased beta-catenin protein levels, and inhibited PI3K/AKT signaling. The inactivation of beta-catenin by beta-catenin siRNA transfection and the activation of PI3K/AKT signaling by p110alpha plasmid transfection abrogated cell death caused by 17-AAG and HMA. CONCLUSION: 17-AAG and HMA have cytotoxic activities accompanied by regulation of HSP90 client proteins, and cytotoxicity is associated with overexpression of beta-catenin and suppression of PI3K/AKT signaling in FRO ATC cells.
Ectopic expression of c-myc fails to overcome downregulation of telomerase activity induced by herbimycin A, but ectopic hTERT expression overcomes it.[Pubmed:26531889]
Leukemia. 2015 Nov;29(11):2274.
Correction to: Leukemia (2000); 14: 1260-1265; doi: 10.1038/sj.leu.2401828. Since the publication of the above article the authors have identified an error in Figure 1. Figure 1 shows the modulation of telomerase activity by Herbimycin A in K562 cells: (a) cell cycle and (b) telomerase activity, mRNA expressions of hTERT, hTERC, TEP-1, c-myc, cyclin D1 and b-actin, and c-Myc protein. The authors however wish to inform the readers that Figure 1b incorrectly shows hTERT mRNA, which is the result of Herbimycin A treatment of cyclin-D1-transfected K562 cells (Figure 3b, hTERT mRNA). While preparing Figure 1, the authors mistakenly submitted a figure that used the incorrect photo data following confusion regarding file names. The correct figure can be found below: The authors wish to apologise for any inconvenience caused and confirm that the conclusions drawn from this research are not affected by this error.
Inhibition of Hsp90 function delays and impairs recovery from heat shock.[Pubmed:16218955]
FEBS J. 2005 Oct;272(20):5244-56.
The induction of the heat shock response as well as its termination is autoregulated by heat shock protein activities. In this study we have investigated whether Hsp90 functional protein levels influence the characteristics and duration of the heat shock response. Treatment of cells with several benzoquinone ansamycin inhibitors of Hsp90 (geldanamycin, Herbimycin A) activated a heat shock response in the absence of heat shock, as reported previously. Pretreatment of cells with the Hsp90 inhibitors significantly delayed the rate of restoration of normal protein synthesis following a brief heat shock. Concurrently, the rate of Hsp synthesis and accumulation was substantially increased and prolonged. The cessation of heat shock protein synthesis did not occur until the levels of Hsp70 were substantially elevated relative to its standard threshold for autoregulation. The elevated levels of HSPS 22-28 (the small HSPS) and Hsp70 are not able to promote thermotolerance when Hsp90 activity is repressed by ansamycins; rather a suppression of thermotolerance is observed. These results suggest that a multicomponent protein chaperone complex involving both Hsp90 and Hsp70 signals the cessation of heat shock protein synthesis, the restoration of normal translation, and likely the establishment of thermotolerance. Impaired function of either component is sufficient to alter the heat shock response.
Herbimycin A inhibits angiogenic activity in endothelial cells and reduces neovascularization in a rat model of retinopathy of prematurity.[Pubmed:15051479]
Exp Eye Res. 2004 May;78(5):987-95.
The pathogenesis of retinopathy of prematurity involves dysregulated angiogenesis resulting in pre-retinal growth of new vessels. Inhibition of tyrosine kinase-dependent pro-angiogenic signals may provide a rational therapeutic approach to the reduction of pre-retinal neovascularization. Vascular endothelial growth factor stimulates endothelial cell mitogenesis, differentiation and migration, by binding and activating the receptor tyrosine kinases vascular endothelial growth factor receptor-1 and vascular endothelial growth factor receptor-2. One of the vascular endothelial growth factor receptor substrates implicated in vascular endothelial growth factor signal transduction is c-Src. The ability of Herbimycin A, a c-Src-selective tyrosine kinase inhibitor, to inhibit vascular endothelial growth factor-induced bovine retinal microvascular endothelial cell proliferation and tube formation was investigated. The ability of the compound to inhibit pathologic angiogenesis was tested in a rat model of retinopathy of prematurity. Exposure of neonatal rats to oxygen concentrations cycling between 10 and 50% induced severe pre-retinal neovascularization in all rats. Some of the eyes of these variable oxygen-exposed rats were Herbimycin A-injected or vehicle-injected 1 or 3 days post-oxygen exposure while some eyes were non-injected. All rats were sacrificed for assessment 6 days post-exposure. Herbimycin A inhibited both vascular endothelial growth factor-induced bovine retinal microvascular endothelial cell proliferation and capillary tube formation in a dose-dependent manner. Injection of Herbimycin A into oxygen-treated rats 1 day post-oxygen exposure produced a 63% decrease in pre-retinal neovascularization relative to vehicle (P = 0.0029). There was a 41% decrease in pre-retinal neovascularization in herbimycin-injected eyes relative to vehicle-injected eyes 3 days post-oxygen (P = 0.031). Pre-retinal neovascularization was reduced in vehicle-injected eyes relative to non-injected eyes at both injection times. There were no significant differences in retinal vascular area between any of the experimental groups. Based on the results of this study, Herbimycin A inhibits endothelial cell proliferation and tube formation at non-toxic concentrations and reduces pre-retinal neovascularization in a rat model of retinopathy of prematurity. Reduction of angiogenesis by the inhibition of tyrosine kinase activity may be a viable route to the development of effective chemotherapies applicable to eye disease.
Labeling of v-Src and BCR-ABL tyrosine kinases with [14C]herbimycin A and its use in the elucidation of the kinase inactivation mechanism.[Pubmed:8131836]
FEBS Lett. 1994 Mar 7;340(3):155-8.
The ansamycin antibiotic, Herbimycin A, selectively inactivates cytoplasmic tyrosine kinases, most likely by binding irreversibly to the reactive SH group(s) of kinases. To further investigate the mechanism of Herbimycin A action, we attempted to label tyrosine kinases with [14C]Herbimycin A. p60v-src and p210BCR-ABL in immune complexes were labeled with [14C]Herbimycin A, demonstrating that the antibiotic binds directly to tyrosine kinases. Digestion of [14C]Herbimycin A-labeled p60v-src with Staphylococcus aureus V8 protease revealed that the Herbimycin A binding site is within the C-terminal 26-kDa fragment of p60v-src, which contains the tyrosine kinase domain. Herbimycin A treatment inhibited labeling of p60v-src by [14C]fluorosulfonylbenzoyl adenosine, an affinity labeling reagent of nucleotide binding sites, indicating that Herbimycin A-modified p60v-src cannot interact with ATP. The results suggest that Herbimycin A inactivates tyrosine kinases by binding directly to the kinase domain, thereby inhibiting access to ATP.


