IsoaltholactoneCAS# 124868-11-7 |
- Altholactone
Catalog No.:BCN4786
CAS No.:65408-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124868-11-7 | SDF | Download SDF |
| PubChem ID | 10399033 | Appearance | Powder |
| Formula | C13H12O4 | M.Wt | 232.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
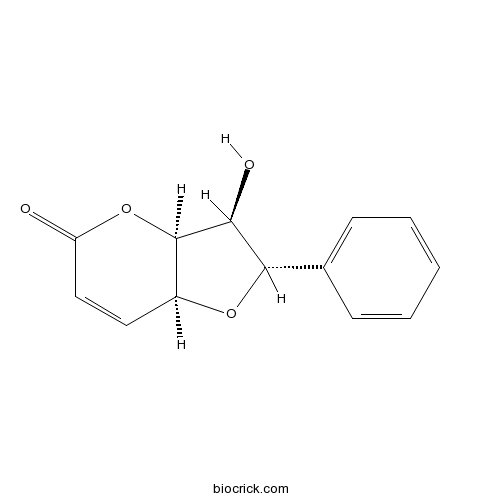
| Chemical Name | (2S,3S,3aS,7aS)-3-hydroxy-2-phenyl-2,3,3a,7a-tetrahydrofuro[3,2-b]pyran-5-one | ||
| SMILES | C1=CC=C(C=C1)C2C(C3C(O2)C=CC(=O)O3)O | ||
| Standard InChIKey | ZKIRVBNLJKGIEM-XYJRDEOASA-N | ||
| Standard InChI | InChI=1S/C13H12O4/c14-10-7-6-9-13(17-10)11(15)12(16-9)8-4-2-1-3-5-8/h1-7,9,11-13,15H/t9-,11-,12-,13+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Tetrahedron Letters.2003 July 28;44(31):5831–5833.A concise and stereoselective synthesis of both enantiomers of altholactone and isoaltholactone.[Reference: WebLink]
European Journal of Organic Chemistry. 2011 Dec; 2011(36).Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction.[Reference: WebLink]
The stereoselective synthesis of nitrogen analogues of biologically active Isoaltholactone and goniothalesdiol are described.
|

Isoaltholactone Dilution Calculator

Isoaltholactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3066 mL | 21.5332 mL | 43.0663 mL | 86.1326 mL | 107.6658 mL |
| 5 mM | 0.8613 mL | 4.3066 mL | 8.6133 mL | 17.2265 mL | 21.5332 mL |
| 10 mM | 0.4307 mL | 2.1533 mL | 4.3066 mL | 8.6133 mL | 10.7666 mL |
| 50 mM | 0.0861 mL | 0.4307 mL | 0.8613 mL | 1.7227 mL | 2.1533 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-Dehydroxyparatocarpin K
Catalog No.:BCN4017
CAS No.:124858-37-3
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Valacyclovir hydrochloride
Catalog No.:BCC4051
CAS No.:124832-27-5
- Valaciclovir
Catalog No.:BCC2025
CAS No.:124832-26-4
- USP7-USP47 inhibitor
Catalog No.:BCC4113
CAS No.:1247825-37-1
- P 22077
Catalog No.:BCC3616
CAS No.:1247819-59-5
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Quinovic acid 3-O-(6-deoxy-beta-D-glucopyranoside) 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1595
CAS No.:124727-10-2
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Methyl-Dodovisate A
Catalog No.:BCN4719
CAS No.:1246937-33-6
- 5'-Prenylaliarin
Catalog No.:BCN4829
CAS No.:1246926-09-9
- 5,7,4'-Trihydroxy-3,6-dimethoxy-3',5'-diprenylflavone
Catalog No.:BCN1596
CAS No.:1246926-08-8
- Benzoyl leuco methylene blue
Catalog No.:BCC8862
CAS No.:1249-97-4
- (R)-(+)-Tolterodine
Catalog No.:BCC4054
CAS No.:124937-51-5
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- Tectoroside
Catalog No.:BCN6672
CAS No.:124960-89-0
- Raddeanoside R8
Catalog No.:BCN6555
CAS No.:124961-61-1
- Ajugacumbin A
Catalog No.:BCN3657
CAS No.:124961-66-6
- Ajugamarin L2
Catalog No.:BCN3662
CAS No.:124961-67-7
- Prednisone 21-acetate
Catalog No.:BCC9128
CAS No.:125-10-0
- Isobornyl acetate
Catalog No.:BCN8296
CAS No.:125-12-2
- Vomicine
Catalog No.:BCN6735
CAS No.:125-15-5
- Primidone
Catalog No.:BCC4930
CAS No.:125-33-7
- Usnic acid
Catalog No.:BCC8264
CAS No.:125-46-2
3-Hy-droxy-2-phenyl-2,3,3a,7a-tetra-hydro-1H,5H-pyrano[3,2-b]pyrrol-5-one: crystal structure and Hirshfeld surface analysis.[Pubmed:28529789]
Acta Crystallogr E Crystallogr Commun. 2017 Apr 21;73(Pt 5):746-751.
The title Isoaltholactone derivative, C13H13NO3, has an NH group in place of the ether-O atom in the five-membered ring of the natural product. The five-membered ring is twisted about the N-C bond linking it to the six-membered ring, which has a half-chair conformation with the O atom connected to the ether-O atom lying above the plane defined by the remaining atoms. The dihedral angle between the mean planes of the rings comprising the fused-ring system is 75.10 (8) degrees . In the crystal, hy-droxy-O-Hcdots, three dots, centeredN(amine) hydrogen bonding sustains linear supra-molecular chains along the a axis. Chains are linked into a three-dimensional architecture via amine-N-Hcdots, three dots, centeredpi(phen-yl) and phenyl-C-Hcdots, three dots, centeredO(hy-droxy) inter-actions. The influence of the amine-N-Hcdots, three dots, centeredpi(phen-yl) contact on the mol-ecular packing is revealed by an analysis of the Hirshfeld surface.
Cobalt-catalyzed diastereoselective synthesis of C-furanosides. Total synthesis of (-)-isoaltholactone.[Pubmed:24127819]
J Org Chem. 2013 Dec 6;78(23):11807-14.
An array of C-aryl and C-vinyl furanosides were prepared in good yields and diastereoselectivities from C-halogeno furanosides either with aryl Grignard or with vinyl Grignard using the convenient Co(acac)3/TMEDA catalytic system. This method is illustrated by the total synthesis of the (-)-Isoaltholactone.
Inhibitory effect of compounds from Goniothalamus tapis Miq. and Goniothalamus uvaroides King on platelet-activating factor receptor binding.[Pubmed:22002630]
Phytother Res. 2012 May;26(5):687-91.
Phytochemical investigation on the bark of Goniothalamus tapis Miq. and G. uvaroides King has resulted in the isolation of eight styryl-lactones, (-)-cryptomeridiol, liriodenine, 3-methyl-1H-benz[f]indole-4,9-dione, (-)-stigmasterol and dimethyl terephthalate. The structures of the compounds were elucidated by spectroscopic techniques. The compounds were evaluated for their effect on platelet-activating factor (PAF) receptor binding on rabbit platelets using (3) H-PAF as a ligand. Among the compounds tested, (-)-cryptomeridiol, (+)-goniothalamin and (+)-Isoaltholactone exhibited a significant and concentration-dependent inhibitory effect on PAF receptor binding, with inhibitory concentration (IC)(50) values of 17.5, 19.7 and 46.5 microm, respectively. The inhibitory effects of the first two compounds were comparable to that obtained from the positive control, cedrol. The results indicated that these compounds were strong PAF receptor binding inhibitors.
Stereospecificity in the Au-catalysed cyclisation of monoallylic diols. Synthesis of (+)-isoaltholactone.[Pubmed:21666896]
Chem Commun (Camb). 2011 Jul 21;47(27):7659-61.
We describe a concise synthesis of (+)-Isoaltholactone via a Au-catalysed cyclisation of a monoallylic diol to form the tetrahydrofuranyl ring. Analogous cyclisations show that the stereochemical outcome is dictated by the stereochemistry of the diol substrate.
A convergent Pd-catalyzed asymmetric allylic alkylation of dl- and meso-divinylethylene carbonate: enantioselective synthesis of (+)-australine hydrochloride and formal synthesis of isoaltholactone.[Pubmed:17847148]
Chemistry. 2007;13(34):9547-60.
The use of a mixture of dl- and meso-divinylethylene carbonate as an electrophile in palladium-catalyzed asymmetric allylic alkylation reactions is reported. From the diastereomeric mixture of meso and chiral racemic starting materials, a single product is obtained in high optical purity employing either oxygen or nitrogen nucleophiles. The resulting dienes have proven to be versatile synthetic intermediates as each carbon is functionalized for further transformation and differentiated by virtue of the reaction. A mechanism for this intriguing transformation is proposed and a concise enantioselective total synthesis of (+)-australine hydrochloride is reported as well as a formal synthesis of Isoaltholactone.
Styryllactones from the rhizomes of Goniothalamus griffithii.[Pubmed:11254031]
J Asian Nat Prod Res. 1999;1(3):189-97.
Three new styryl-lactones 8-acetylgoniofufurone(1), 7-acetylgonio-pypyrone(3), and 5-acetylgoniopypyrone(4), along with ten known compounds, goniofufurone(2), goniopypyrone(5), goniothalamin, goniothalenol, (+)-Isoaltholactone, goniodiol, 7-acetylgoniodiol, goniotriol, 8-acetylgoniotriol, 9-deoxygoniopypyrone were isolated from the rhizomes of Goniothalamus griffithii Hook f. et. Thoms. Their structures were elucidated by IR, MS, NMR spectra and chemical evidence. All compounds showed cytotoxic activities against human cancer cell lines.
Enantioselective syntheses of isoaltholactone, 3-epi-altholactone, and 5-hydroxygoniothalamin.[Pubmed:10986088]
Org Lett. 2000 Sep 21;2(19):2983-6.
A flexible enantioselective route to highly functionalized alpha, beta-unsaturated delta-lactones has allowed for the syntheses of the styryllactones: Isoaltholactone, 3-epi-altholactone, and 5-hydroxygoniothalamin in 10%, 5%, and 13% overall yields from furfural, respectively. This approach derives its asymmetry by applying the Sharpless catalytic asymmetric dihydroxylation to vinylfuran. The resulting diols are produced in high enantioexcess and can be stereoselectively transformed into alpha,beta-unsaturated delta-lactones via a short highly diastereoselective oxidation and reduction sequence.


