USP7-USP47 inhibitorUSP7/USP47 inhibitor CAS# 1247825-37-1 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1247825-37-1 | SDF | Download SDF |
| PubChem ID | 51031028 | Appearance | Powder |
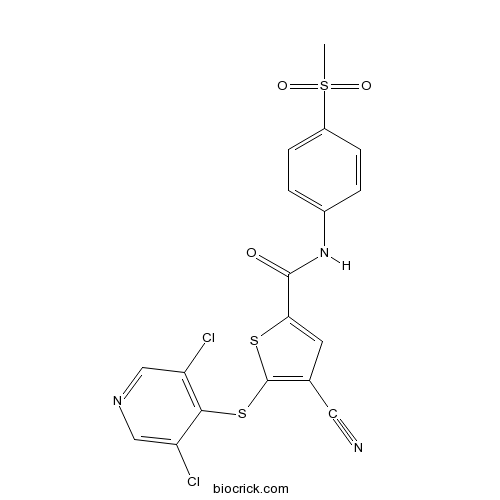
| Formula | C18H11Cl2N3O3S3 | M.Wt | 484.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (103.22 mM; Need ultrasonic) | ||
| Chemical Name | 4-cyano-5-(3,5-dichloropyridin-4-yl)sulfanyl-N-(4-methylsulfonylphenyl)thiophene-2-carboxamide | ||
| SMILES | CS(=O)(=O)C1=CC=C(C=C1)NC(=O)C2=CC(=C(S2)SC3=C(C=NC=C3Cl)Cl)C#N | ||
| Standard InChIKey | GUDJFFQZIISQJB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H11Cl2N3O3S3/c1-29(25,26)12-4-2-11(3-5-12)23-17(24)15-6-10(7-21)18(27-15)28-16-13(19)8-22-9-14(16)20/h2-6,8-9H,1H3,(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | USP7/USP47 inhibitor is a selective ubiquitin-specific protease 7/47 (USP7/USP47) inhibitor, with EC50s of 0.42 μM and 1.0 μM, respectively.In Vitro:USP7/USP47 inhibitor (compound 14) is a selective inhibitor of USP7/USP47 with EC50s of 0.42 μM and 1 μM, respectively. USP7/USP47 inhibitor does not inhibit caspase 3, calpain 1, 20S proteasome, and a panel of representative USPs (USP2, USP5, USP8, USP21, and USP28; EC50 > 31.6 μM). USP7/USP47 inhibitor inhibits the growth of HCT-116 cells with an EC50 of 7.6 μM[1]. References: | |||||

USP7-USP47 inhibitor Dilution Calculator

USP7-USP47 inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0644 mL | 10.322 mL | 20.6441 mL | 41.2882 mL | 51.6102 mL |
| 5 mM | 0.4129 mL | 2.0644 mL | 4.1288 mL | 8.2576 mL | 10.322 mL |
| 10 mM | 0.2064 mL | 1.0322 mL | 2.0644 mL | 4.1288 mL | 5.161 mL |
| 50 mM | 0.0413 mL | 0.2064 mL | 0.4129 mL | 0.8258 mL | 1.0322 mL |
| 100 mM | 0.0206 mL | 0.1032 mL | 0.2064 mL | 0.4129 mL | 0.5161 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
USP7/USP47 Inhibitor(P 22077)is a selective and dual inhibitor of human ubiquitin-specific proteases 7 (USP7) and 47(USP47) with EC50 values of 4.2±0.9μM and 4.3±0.8μM, respectively [1].
USP7/USP47 Inhibitor is a selective inhibitor of USP7 as well as USP47 and shows little or no inhibition activity for many USP variants or other classes of protease, including caspase, cathepsins, calpain, metalloproteases and serine proteases. In addition, USP7/USP47 Inhibitor has been reported to accelerate the degradation of the USP7 substrate HDM2 in cancer cell lines. Besides, USP7/USP47 Inhibitor has been revealed to inhibit the growth of HCT-116 human colorectal cancer cells with an EC50 value of 11μM. Moreover, USP7/USP47 Inhibitor has also been noted to significantly enhance the lifespan and lower the tumor burden of mice in human multiple myeloma and B cell leukemia xenograft models [1].
References:
[1] Weinstock J, Wu J, Cao P, Kingsbury WD, McDermott JL, Kodrasov MP, McKelvey DM, Suresh Kumar KG, Goldenberg SJ, Mattern MR, Nicholson B.Selective Dual Inhibitors of the Cancer-Related Deubiquitylating Proteases USP7 and USP47.ACS Med Chem Lett. 2012 Sep 11;3(10):789-92.
- P 22077
Catalog No.:BCC3616
CAS No.:1247819-59-5
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Quinovic acid 3-O-(6-deoxy-beta-D-glucopyranoside) 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1595
CAS No.:124727-10-2
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Methyl-Dodovisate A
Catalog No.:BCN4719
CAS No.:1246937-33-6
- 5'-Prenylaliarin
Catalog No.:BCN4829
CAS No.:1246926-09-9
- 5,7,4'-Trihydroxy-3,6-dimethoxy-3',5'-diprenylflavone
Catalog No.:BCN1596
CAS No.:1246926-08-8
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- MPI-0479605
Catalog No.:BCC5347
CAS No.:1246529-32-7
- SR1078
Catalog No.:BCC1963
CAS No.:1246525-60-9
- (-)-Dihydroguaiaretic acid
Catalog No.:BCN8002
CAS No.:124649-78-1
- Officinaruminane B
Catalog No.:BCN3594
CAS No.:1246282-67-6
- Valaciclovir
Catalog No.:BCC2025
CAS No.:124832-26-4
- Valacyclovir hydrochloride
Catalog No.:BCC4051
CAS No.:124832-27-5
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- 5-Dehydroxyparatocarpin K
Catalog No.:BCN4017
CAS No.:124858-37-3
- Isoaltholactone
Catalog No.:BCN4826
CAS No.:124868-11-7
- Benzoyl leuco methylene blue
Catalog No.:BCC8862
CAS No.:1249-97-4
- (R)-(+)-Tolterodine
Catalog No.:BCC4054
CAS No.:124937-51-5
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- Tectoroside
Catalog No.:BCN6672
CAS No.:124960-89-0
- Raddeanoside R8
Catalog No.:BCN6555
CAS No.:124961-61-1
- Ajugacumbin A
Catalog No.:BCN3657
CAS No.:124961-66-6
- Ajugamarin L2
Catalog No.:BCN3662
CAS No.:124961-67-7
Chemical Synthesis and Evaluation of a Disialic Acid-Containing Dextran Polymer as an Inhibitor for the Interaction between Siglec 7 and Its Ligand.[Pubmed:28374980]
Chembiochem. 2017 Jul 4;18(13):1194-1203.
A new sialic acid (Sia)-containing glycopolymer-a fluorescent probe with high-density disialic acid (diSia) on the surface of polysaccharide dextran (diSia-Dex)-was synthesized as a key molecule to regulate the Sia recognition lectins, Siglecs, that are involved in the immune system. According to our original methods, diSia was synthesized by alpha-selective sialylation, and a dextran template possessing terminal acetylenes and amino groups was prepared. A diSia and a fluorescent molecule were subsequently introduced to surface-modified dextran by Huisgen reaction and amidation, respectively. The modulatory activity of Siglec7 was evaluated by using synthetic probes. DiSia-Dex showed high binding avidity toward Siglec7, with a KD value of 5.87x10(-10) m, and a high inhibitory activity for the interaction between Siglec7 and a ligand (GD3), with a IC50 value of 1.0 nm. Notably, diSia-Dex was able to release Siglec7 from the pre-existing Siglec7-GD3 complex, possibly due to its unique properties of a slow dissociation rate and a high association rate. Together, these data show that diSia-Dex can be widely applicable as a modulator of Siglec7 functions.
Durable Control of Metastatic AKT1-Mutant WHO Grade 1 Meningothelial Meningioma by the AKT Inhibitor, AZD5363.[Pubmed:28376212]
J Natl Cancer Inst. 2017 Mar 1;109(3):1-4.
High-throughput analyses have revealed the presence of activating mutations in the AKT1 gene in a subpopulation of meningiomas. We report a female patient with multiple intracranial tumor manifestations and histologically verified meningotheliomatous meningioma in the lung. The tumor was continuously growing at multiple sites despite six surgical resections, radiotherapy, and two lines of systemic therapy. Following detection of an AKT1E17K mutation in three independent tumor samples by sequencing, treatment with AZD5363, a selective AKT inhibitor, was initiated. Ex vivo cultured meningioma cells exhibited sensitivity to the drug as shown by pAKT accumulation on immunoblots. Treatment with AZD5363 resulted, for the first time, in stable disease and minor radiographic response. The patient has been on that treatment for more than one year with ongoing clinical and radiographic response. This is the first report of an AKT1-mutant meningioma responding to AKT inhibition, suggesting that molecular screening may result in clinical benefit.
Emergent drug resistance with integrase strand transfer inhibitor-based regimens.[Pubmed:28375875]
AIDS. 2017 Jun 19;31(10):1425-1434.
OBJECTIVES: To estimate the incidence of and risk factors for emergent resistance to integrase strand transfer inhibitor (INSTI) and nucleoside(-tide) reverse transcriptase inhibitors (NRTI) in HIV-1-infected adults receiving an INSTI and two NRTIs. DESIGN: Retrospective cohort study. METHODS: Persons aged at least 19 years were included if they received their first prescription for raltegravir, elvitegravir or dolutegravir in British Columbia, Canada in 2012-2014 and were followed to 31 December 2015. Emergent resistance was defined as new mutations conferring intermediate-high level NRTI or INSTI resistance (score >/=30, Stanford HIV Drug Resistance Algorithm v.7.0.1). First-year resistance rates and 95% confidence intervals (95% CI) were estimated for 'any' (INSTI or NRTI) resistance using Poisson regression. The relationship between any emergent resistance and explanatory variables was modeled by Cox proportional hazards. RESULTS: There were 270 raltegravir, 323 elvitegravir and 392 dolutegravir-treated persons who were predominantly male (77%), antiretroviral therapy (ART)-experienced (81%), with low prevalence of preexisting drug resistance (16%). INSTI and NRTI resistance emerged in both ART-experienced and ART-naive persons (including dolutegravir-treated ART-naive), with no statistically significant differences in 'any' resistance rates (95% CI) between INSTIs: raltegravir 3.80 (1.90, 7.60), elvitegravir 2.37 (1.06, 5.27) and dolutegravir 1.48 (0.62, 3.55)/100 person-years. The strongest factors associated with emergent resistance were CD4 less than 200 cells/mul, adjusted hazard ratio (95% CI) 10.46 (4.67, 23.41) and less than 80% adherence to the INSTI regimen hazard ratio 2.52 (1.11, 5.71). CONCLUSION: Incident drug resistance rates were low with 'real-world' use of INSTI-based regimens. However, incomplete ART adherence and low CD4 cell count were associated with increased resistance rates regardless of which INSTI was prescribed. Provide adherence support and monitor for drug resistance.
Rad51 Degradation: Role in Oncolytic Virus-Poly(ADP-Ribose) Polymerase Inhibitor Combination Therapy in Glioblastoma.[Pubmed:28376211]
J Natl Cancer Inst. 2017 Mar 1;109(3):1-13.
Background: Clinical success of poly(ADP-ribose) polymerase inhibitors (PARP i ) has been limited to repair-deficient cancers and by resistance. Oncolytic herpes simplex viruses (oHSVs) selectively kill cancer cells, irrespective of mutation, and manipulate DNA damage responses (DDR). Here, we explore potential synthetic lethal-like interactions between oHSV and PARP i . Methods: The efficacy of combining PARP i , oHSV MG18L, and G47Delta in killing patient-derived glioblastoma stem cells (GSCs) was assessed using cell viability assays and Chou-Talalay synergy analysis. Effects on DDR pathways, apoptosis, and cell cycle after manipulation with pharmacological inhibitors and lentivirus-mediated knockdown or overexpression were examined by immunoblotting and FACS. In vivo efficacy was evaluated in two GSC-derived orthotopic xenograft models (n = 7-8 per group). All statistical tests were two-sided. Results: GSCs are differentially sensitive to PARP i despite uniform inhibition of PARP activity. oHSV sensitized GSCs to PARP i , irrespective of their PARP i sensitivity through selective proteasomal degradation of key DDR proteins; Rad51, mediating the combination effects; and Chk1. Rad51 degradation required HSV DNA replication. This synthetic lethal-like interaction increased DNA damage, apoptosis, and cell death in vitro and in vivo. Combined treatment of mice bearing PARP i -sensitive or -resistant GSC-derived brain tumors greatly extended median survival compared to either agent alone (vs olaparib: P


