IsobiflorinCAS# 152041-16-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152041-16-2 | SDF | Download SDF |
| PubChem ID | 10338198 | Appearance | Powder |
| Formula | C16H18O9 | M.Wt | 354.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
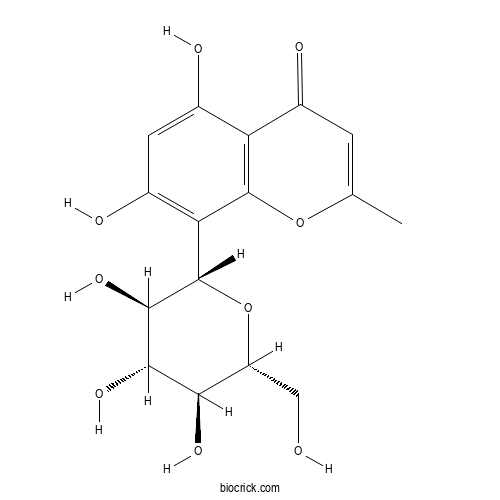
| Chemical Name | 5,7-dihydroxy-2-methyl-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | ||
| SMILES | CC1=CC(=O)C2=C(O1)C(=C(C=C2O)O)C3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | UDTUCCXZNVRBEJ-PBVFJORSSA-N | ||
| Standard InChI | InChI=1S/C16H18O9/c1-5-2-6(18)10-7(19)3-8(20)11(15(10)24-5)16-14(23)13(22)12(21)9(4-17)25-16/h2-3,9,12-14,16-17,19-23H,4H2,1H3/t9-,12-,13+,14-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isobiflorin Dilution Calculator

Isobiflorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8225 mL | 14.1123 mL | 28.2247 mL | 56.4493 mL | 70.5617 mL |
| 5 mM | 0.5645 mL | 2.8225 mL | 5.6449 mL | 11.2899 mL | 14.1123 mL |
| 10 mM | 0.2822 mL | 1.4112 mL | 2.8225 mL | 5.6449 mL | 7.0562 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5645 mL | 1.129 mL | 1.4112 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5645 mL | 0.7056 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bracteanolide A
Catalog No.:BCX2080
CAS No.:1021432-39-2
- Graciliflorin E
Catalog No.:BCX2079
CAS No.:1413941-66-8
- 6,12,15-Trihydroxy-5,8,11,13-abietetra-7-one
Catalog No.:BCX2078
CAS No.:371155-09-8
- Gerardianin B
Catalog No.:BCX2077
CAS No.:2070919-73-0
- 16-Acetoxylsugiol
Catalog No.:BCX2076
CAS No.:1413941-65-7
- Aesculitannin B
Catalog No.:BCX2075
CAS No.:114612-77-0
- MTCA(1-Methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid)
Catalog No.:BCX2074
CAS No.:5470-37-1
- Strychnistenolide 6-O-acetate
Catalog No.:BCX2073
CAS No.:325969-56-0
- 4'''-O-beta-D-Glucopyranosyltrifloroside
Catalog No.:BCX2072
CAS No.:188350-29-0
- Azalein
Catalog No.:BCX2071
CAS No.:29028-02-2
- Epicatechin 3-O-(4-O-methylgallate)
Catalog No.:BCX2070
CAS No.:108907-44-4
- 15-Hydroxy-7-oxo-abieta-8,11,13-triene
Catalog No.:BCX2069
CAS No.:105037-83-0
- 2-Hydroxy-6-methoxy-4-O-(6''-O-alpha-L-arabinofuranosyl-beta-D-glucopyranosyl)acetophenone
Catalog No.:BCX2082
CAS No.:1452160-87-0
- Fischeroside A
Catalog No.:BCX2083
CAS No.:1307257-07-3
- 16-Acetoxy-7alpha-methoxyroyleanone
Catalog No.:BCX2084
CAS No.:109974-33-6
- Methyl-6-gingerol
Catalog No.:BCX2085
CAS No.:23513-10-2
- Rhamnetin 3-O-gentiobioside
Catalog No.:BCX2086
CAS No.:1786990-15-8
- Rhamnetin 3-sophoroside
Catalog No.:BCX2087
CAS No.:259234-17-8
- Suksdorfin
Catalog No.:BCX2088
CAS No.:53023-17-9
- Notoginsenoside SFt4
Catalog No.:BCX2089
CAS No.:1351360-30-9
- Albanin D
Catalog No.:BCX2090
CAS No.:134955-26-3
- 4'-O-Methylisoliquiritigenin
Catalog No.:BCX2091
CAS No.:476487-22-6
- Rutarin
Catalog No.:BCX2092
CAS No.:20320-81-4
- 5'-Methoxyoctahydrocurcumin
Catalog No.:BCX2093
CAS No.:718638-77-8
Cancer therapeutic potential of hovetrichoside C from Jatropha podagrica on apoptosis of MDA-MB-231 human breast cancer cells.[Pubmed:38849046]
Food Chem Toxicol. 2024 Aug;190:114794.
Phytochemical analysis of the methanolic extracts of Jatropha podagrica stalks and roots using liquid chromatography-mass spectrometry (LC-MS) led to the isolation of six compounds: corchoionoside C (1), Isobiflorin (2), fraxin (3), hovetrichoside C (4), fraxetin (5), and corillagin (6). The isolated compounds (1-6) were tested for their cytotoxicity against MDA-MB-231 human breast cancer cells. Remarkably, compound 4 (hovetrichoside C) exhibited robust cytotoxicity against MDA-MB-231 cells, displaying an IC(50) value of 50.26 +/- 1.22 muM, along with an apoptotic cell death rate of 24.21 +/- 2.08% at 100 muM. Treatment involving compound 4 amplified protein levels of cleaved caspase-8, -9, -3, -7, BH3-interacting domain death agonist (Bid), Bcl-2-associated X protein (Bax), and cleaved poly (ADP-ribose) polymerase (cleaved PARP), while concurrently reducing B-cell lymphoma 2 (Bcl-2) levels. In totality, these findings underscore that hovetrichoside C (4) possesses anti-breast cancer activity that revolves around apoptosis induction via both extrinsic and intrinsic signaling pathways.
A new isoflavone glycoside from Abrus cantoniensis.[Pubmed:30982343]
J Asian Nat Prod Res. 2020 Jun;22(6):588-593.
A new isoflavone glycoside named as 8-O-methylrelusin-7-O-beta-D-apifuranosyl-(1-->2)-beta-D-glucopyranoside (1), together with two known compounds, 8-O-methylrelusin-7-O-beta-D-glucopyranoside (2) and Isobiflorin (3), were isolated from Abrus cantoniensis. The structure of the new compound was elucidated on the basis of spectroscopic methods including extensive 1D NMR, 2D NMR, and HRESIMS. This is the first report of isoflavone from Abrus cantoniensis. Moreover, all isolated compounds were evaluated for their cytotoxicity against SMMC-7721 and MHCC97-H cell lines.[Formula: see text].
Biflorin, Isolated from the Flower Buds of Syzygium aromaticum L., Suppresses LPS-Induced Inflammatory Mediators via STAT1 Inactivation in Macrophages and Protects Mice from Endotoxin Shock.[Pubmed:26977531]
J Nat Prod. 2016 Apr 22;79(4):711-20.
Two chromone C-glucosides, biflorin (1) and Isobiflorin (2), were isolated from the flower buds of Syzygium aromaticum L. (Myrtaceae). Here, inhibitory effects of 1 and 2 on lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in RAW 264.7 macrophages were evaluated, and 1 (IC50 = 51.7 and 37.1 muM, respectively) was more potent than 2 (IC50 > 60 and 46.0 muM). The suppression of NO and PGE2 production by 1 correlated with inhibition of iNOS and COX-2 protein expression. Compound 1 reduced inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) mRNA expression via inhibition of their promoter activities. Compound 1 inhibited the LPS-induced production and mRNA expression of tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-6. Furthermore, 1 reduced p-STAT1 and p-p38 expression but did not affect the activity of nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) or activator protein 1 (AP-1). In a mouse model of LPS-induced endotoxemia, 1 reduced the mRNA levels of iNOS, COX-2, and TNF-alpha, and the phosphorylation-mediated activation of the signal transducer and activator of transcription 1 (STAT1), consequently improving the survival rates of mice. Compound 1 showed a significant anti-inflammatory effect on carrageenan-induced paw edema and croton-oil-induced ear edema in rats. The collective data indicate that the suppression of pro-inflammatory gene expression via p38 mitogen-activated protein kinase and STAT1 inactivation may be a mechanism for the anti-inflammatory activity of 1.
Phenolic constituents from the rhizomes of Dryopteris crassirhizoma.[Pubmed:16651784]
Chem Pharm Bull (Tokyo). 2006 May;54(5):748-50.
A new phenolic glycoside, dryopteroside (1), was isolated from the rhizomes of Dryopteris crassirhizoma (Dryopteridaceae), together with five known compounds, 4beta-carboxymethyl-(-)-epicatechin (2), Isobiflorin (3), biflorin (4), 1-beta-D-glucopyranosyloxy-3-methoxy-5-hydroxybenzene (5) and (+)-catechin-6-C-beta-D-glucopyranoside (6). The new compound was elucidated to be 1-butanoyl-3-C-beta-D-glucopyranosyl-5-methyl-phloroglucinyl-6-O-beta-D-glucopyranoside (1) by chemical and various spectroscopic analyses. The known compounds 2-6 were first reported from the genus Dryopteris.
Isolation of virus-cell fusion inhibitory components from Eugenia caryophyllata.[Pubmed:11345703]
Planta Med. 2001 Apr;67(3):277-9.
The fractionation of Eugenia caryophyllata (Myrtaceae) guided by the syncytia formation inhibition assay led to the isolation of four tannins (eugeniin, casuarictin, 1,3-di-O-galloyl-4,6-(S)-hexahydroxydiphenoyl-beta-D-glucopyranose, and tellimagrandin I), and two chromones (biflorin and Isobiflorin). Among the isolated compounds, tellimagrandin (4) showed a significantly high inhibitory activity on the syncytia formation with an IC50 value of 16.12 +/- 1.98 micrograms/ml.


