SuksdorfinCAS# 53023-17-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53023-17-9 | SDF | Download SDF |
| PubChem ID | 72414 | Appearance | Powder |
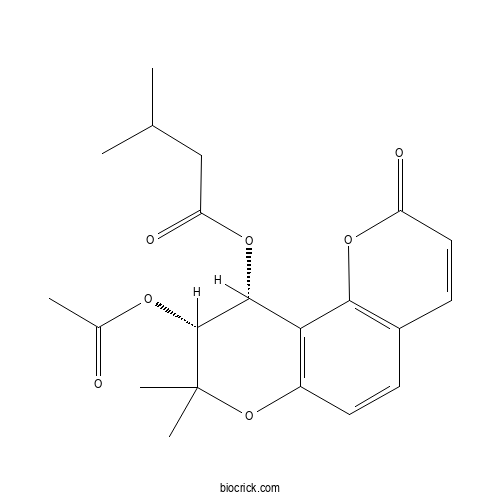
| Formula | C21H24O7 | M.Wt | 388.4 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(9R,10R)-9-acetyloxy-8,8-dimethyl-2-oxo-9,10-dihydropyrano[2,3-f]chromen-10-yl] 3-methylbutanoate | ||
| SMILES | CC(C)CC(=O)OC1C(C(OC2=C1C3=C(C=C2)C=CC(=O)O3)(C)C)OC(=O)C | ||
| Standard InChIKey | KLUTZDJBVDPOFE-WOJBJXKFSA-N | ||
| Standard InChI | InChI=1S/C21H24O7/c1-11(2)10-16(24)27-19-17-14(28-21(4,5)20(19)25-12(3)22)8-6-13-7-9-15(23)26-18(13)17/h6-9,11,19-20H,10H2,1-5H3/t19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Suksdorfin Dilution Calculator

Suksdorfin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5747 mL | 12.8733 mL | 25.7467 mL | 51.4933 mL | 64.3666 mL |
| 5 mM | 0.5149 mL | 2.5747 mL | 5.1493 mL | 10.2987 mL | 12.8733 mL |
| 10 mM | 0.2575 mL | 1.2873 mL | 2.5747 mL | 5.1493 mL | 6.4367 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.5149 mL | 1.0299 mL | 1.2873 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2575 mL | 0.5149 mL | 0.6437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rhamnetin 3-sophoroside
Catalog No.:BCX2087
CAS No.:259234-17-8
- Rhamnetin 3-O-gentiobioside
Catalog No.:BCX2086
CAS No.:1786990-15-8
- Methyl-6-gingerol
Catalog No.:BCX2085
CAS No.:23513-10-2
- 16-Acetoxy-7alpha-methoxyroyleanone
Catalog No.:BCX2084
CAS No.:109974-33-6
- Fischeroside A
Catalog No.:BCX2083
CAS No.:1307257-07-3
- 2-Hydroxy-6-methoxy-4-O-(6''-O-alpha-L-arabinofuranosyl-beta-D-glucopyranosyl)acetophenone
Catalog No.:BCX2082
CAS No.:1452160-87-0
- Isobiflorin
Catalog No.:BCX2081
CAS No.:152041-16-2
- Bracteanolide A
Catalog No.:BCX2080
CAS No.:1021432-39-2
- Graciliflorin E
Catalog No.:BCX2079
CAS No.:1413941-66-8
- 6,12,15-Trihydroxy-5,8,11,13-abietetra-7-one
Catalog No.:BCX2078
CAS No.:371155-09-8
- Gerardianin B
Catalog No.:BCX2077
CAS No.:2070919-73-0
- 16-Acetoxylsugiol
Catalog No.:BCX2076
CAS No.:1413941-65-7
- Notoginsenoside SFt4
Catalog No.:BCX2089
CAS No.:1351360-30-9
- Albanin D
Catalog No.:BCX2090
CAS No.:134955-26-3
- 4'-O-Methylisoliquiritigenin
Catalog No.:BCX2091
CAS No.:476487-22-6
- Rutarin
Catalog No.:BCX2092
CAS No.:20320-81-4
- 5'-Methoxyoctahydrocurcumin
Catalog No.:BCX2093
CAS No.:718638-77-8
- 6-Hydroxykaempferol 7-glucoside
Catalog No.:BCX2094
CAS No.:70056-55-2
- Isosyringalide 3'-rhamnoside
Catalog No.:BCX2095
CAS No.:110326-99-3
- Tupichinol B
Catalog No.:BCX2096
CAS No.:497142-89-9
- Isorutarin
Catalog No.:BCX2097
CAS No.:53846-51-8
- Wattigenin A
Catalog No.:BCX2098
CAS No.:171485-66-8
- 4-(3,5-Dihydroxy-7-(4-hydroxy-3-methoxyphenyl)heptyl)benzene-1,2-diol
Catalog No.:BCX2099
CAS No.:884495-94-7
- 2-Methoxy-2-methyl-6-(4-methylphenyl)-4-heptanone
Catalog No.:BCX2100
CAS No.:70369-29-8
Suksdorfin Promotes Adipocyte Differentiation and Improves Abnormalities in Glucose Metabolism via PPARgamma Activation.[Pubmed:28601955]
Lipids. 2017 Jul;52(7):657-664.
Although the Apiaceae herb family has been traditionally used for the management of type 2 diabetes, its molecular mechanism has not been clarified. Coumarin derivatives, which are abundant in plants of the Apiaceae family, were evaluated for their effects on adipogenesis. We found that Suksdorfin significantly promoted adipocyte differentiation and enhanced production of adiponectin, an anti-diabetic adipokine. We also demonstrated that Suksdorfin activates peroxisome proliferator-activated receptor gamma (PPARgamma), a master regulator of adipogenesis. Furthermore, we showed metabolic disorders in obese diabetic KK-A(y) mice were attenuated by Suksdorfin feeding. Suksdorfin intake induced adipocyte miniaturization and increased expression levels of PPARgamma target genes related to adipocyte differentiation. These results indicated that Suksdorfin induces adipogenesis in white adipose tissue (WAT) via the activation of PPARgamma, leading to improvement of obesity-induced metabolic disorders. Therefore, Suksdorfin-mediated amelioration of WAT dysfunctions might be responsible for the anti-diabetic effects of traditional herbal medicine therapy with Apiaceae.
Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach.[Pubmed:20187635]
J Nat Prod. 2010 Mar 26;73(3):500-16.
Medicinal plants have long been an excellent source of pharmaceutical agents. Accordingly, the long-term objectives of the author's research program are to discover and design new chemotherapeutic agents based on plant-derived compound leads by using a medicinal chemistry approach, which is a combination of chemistry and biology. Different examples of promising bioactive natural products and their synthetic analogues, including sesquiterpene lactones, quassinoids, naphthoquinones, phenylquinolones, dithiophenediones, neo-tanshinlactone, tylophorine, Suksdorfin, DCK, and DCP, will be presented with respect to their discovery and preclinical development as potential clinical trial candidates. Research approaches include bioactivity- or mechanism of action-directed isolation and characterization of active compounds, rational drug design-based modification and analogue synthesis, and structure-activity relationship and mechanism of action studies. Current clinical trial agents discovered by the Natural Products Research Laboratories, University of North Carolina, include bevirimat (dimethyl succinyl betulinic acid), which is now in phase IIb trials for treating AIDS. Bevirimat is also the first in a new class of HIV drug candidates called "maturation inhibitors". In addition, an etoposide analogue, GL-331, progressed to anticancer phase II clinical trials, and the curcumin analogue JC-9 is in phase II clinical trials for treating acne and in development for trials against prostate cancer. The discovery and development of these clinical trial candidates will also be discussed.
Inhibitory effects of coumarin and acetylene constituents from the roots of Angelica furcijuga on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages.[Pubmed:16226032]
Bioorg Med Chem. 2006 Jan 15;14(2):456-63.
The methanolic extract (200 mg/kg, p.o. and i.p.), principal coumarin constituents (isoepoxypteryxin, anomalin, and praeroside IV), and a polyacetylene constituent (falcarindiol) (25 mg/kg, i.p.) from the roots of Angelica furcijuga protected the liver injury induced by D-galactosamine (D-GalN)/lipopolysaccharide (LPS) in mice. In in vitro experiments, coumarin constituents (hyuganins A-D, anomalin, pteryxin, isopteryxin, and Suksdorfin) and polyacetylene constituents [(-)-falcarinol and falcarindiol] substantially inhibited LPS-induced NO and/or TNF-alpha production in mouse peritoneal macrophages, and isoepoxypteryxin inhibited D-GalN-induced cytotoxicity in primary cultured rat hepatocytes. Furthermore, hyuganin A, anomalin, and isopteryxin inhibited the decrease in cell viability by TNF-alpha in L929 cells.
Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga.[Pubmed:9873511]
Bioorg Med Chem Lett. 1998 Aug 18;8(16):2191-6.
The methanolic extract from the roots of Angelica furcijuga KITAGAWA was found to exhibit protective effects on liver injury induced by D-galactosamine (D-GalN) and lipopolysaccharide (LPS). From the methanolic extract, seventeen coumarins, two phenylpropanoids, and two polyacetylenes were isolated and examined their in vitro and in vivo hepatoprotective effects and inhibitory activity of NO production in macrophages. A acylated khellactone, isoepoxypteryxin, showed protective activity against D-GalN-induced cytotoxicity in primary cultured rat hepatocytes. On the other hand, six acylated khellactones (hyuganins A, B, C, and D, anomalin, isopteryxin) and two polyacetylenes [(-)-falcarinol and falcarindiol] strongly inhibited NO production induced by LPS in cultured mouse peritoneal macrophages, and also other acylated khellactones (isoepoxypteryxin, pteryxin, and Suksdorfin) and a coumarin glycosides (praeroside II) were found to show the activity. By comparison of the inhibitory activities for acylated khellactones with those for other coumarins, acyl groups were found to be essential to exerting potent activity.
Anti-AIDS agents. 15. Synthesis and anti-HIV activity of dihydroseselins and related analogs.[Pubmed:7525962]
J Med Chem. 1994 Nov 11;37(23):3947-55.
Forty-two dihydroseselins based on the structure of Suksdorfin (1) were synthesized in order to evaluate their anti-HIV activity. These synthetic derivatives include 3',4'-di-O-acyl- and 3'- or 4'-O-acyl-cis-dihydroseselins (8-21) and 3',4'-trans-dihydroseselins with O-acyl and/or O-alkyl groups at the 3' and 4' positions (6, 22-43). Two 4'-azido (44, 45) and three 4'-alkylamido (46, 48, 49) derivatives were also prepared. By using optically pure reagents, three pairs of diastereoisomers were synthesized and separated as optically pure compounds (14, 15; 16, 17; 38, 39). Together with the above synthetic derivatives, seselin (3) and (+/-)-cis-(4), (+)-cis- (5), and (+/-)-trans-dihydroseselin-3',4'-diol (7) were also tested for their in vitro anti-HIV activity. An optically pure compound, 3',4'-di-O-(-)-camphanoyl-(+)-cis-khellactone (16), showed potent inhibitory activity and remarkable selectivity against HIV replication. The EC50 value and in vitro therapeutic index (TI) of 16 are 4 x 10(-4) microM and 136,719, respectively, which are better than those shown by AZT in the same assay. In addition, compound 16 is also active against HIV replication in a monocytic cell line and in peripheral blood mononuclear cells (PBMCs). Our in vitro assay indicated that, like compound 1, compound 16 is not an inhibitor of HIV-1 reverse transcriptase. Moreover, the anti-HIV activity of 16 is stereoselective as its three diastereoisomers (17, 38, 39) are at least 10,000 times less active. Since other synthetic dihydroseselin derivatives with different substituents or without any substituents are inactive or are active only at much higher concentration, the antiviral potency of 16 could be associated with the camphanoyl moieties of its structure. Therefore, compound 16 represents a unique coumarin structure with promising anti-HIV activity.
Suksdorfin: an anti-HIV principle from Lomatium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides.[Pubmed:7773621]
Bioorg Med Chem. 1994 Oct;2(10):1051-6.
Suksdorfin (1), which is isolated from the fruit of Lomatium suksdorfii, was found to be able to inhibit HIV-1 replication in the T cell line, H9, with an average EC50 value of 2.6 +/- 2.1 microM. In addition, Suksdorfin was also suppressive during acute HIV-1 infections of peripheral blood mononuclear cells, monocyte/macrophages and the promonocytic cell line, U937. Combinations of 1 and the anti-HIV nucleosides ddI and ddC demonstrated statistical synergy in inhibiting HIV-1 replication (ddC > ddI). However, the viral inhibition mediated by combining 1 with AZT was not statistically synergistic. Furthermore, the presence of Suksdorfin did not antagonize the suppression mediated by the three nucleoside reverse transcriptase inhibitors. Comparison of the structure and activity of 1 with those of ten related compounds indicated that the dihydroseselin type of pyranocoumarin possessing a 4'-isovaleryl group is important to Suksdorfin's enhanced anti-HIV activity.


