RutarinCAS# 20320-81-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20320-81-4 | SDF | Download SDF |
| PubChem ID | 442149 | Appearance | Powder |
| Formula | C20H24O10 | M.Wt | 424.4 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
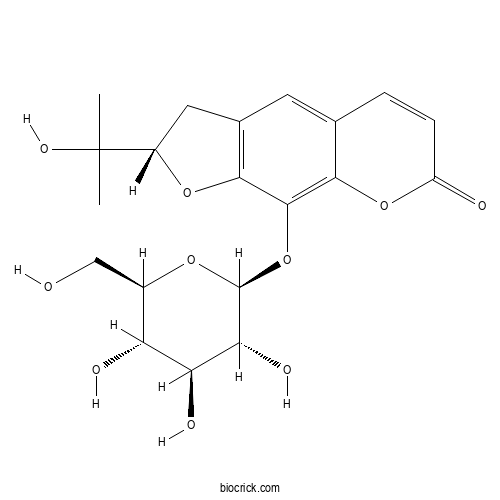
| Chemical Name | (2S)-2-(2-hydroxypropan-2-yl)-9-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2,3-dihydrofuro[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C1CC2=C(O1)C(=C3C(=C2)C=CC(=O)O3)OC4C(C(C(C(O4)CO)O)O)O)O | ||
| Standard InChIKey | JWWFVRMFYKPZNE-VVIWCBLHSA-N | ||
| Standard InChI | InChI=1S/C20H24O10/c1-20(2,26)11-6-9-5-8-3-4-12(22)29-16(8)18(17(9)28-11)30-19-15(25)14(24)13(23)10(7-21)27-19/h3-5,10-11,13-15,19,21,23-26H,6-7H2,1-2H3/t10-,11+,13-,14+,15-,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rutarin Dilution Calculator

Rutarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3563 mL | 11.7813 mL | 23.5627 mL | 47.1254 mL | 58.9067 mL |
| 5 mM | 0.4713 mL | 2.3563 mL | 4.7125 mL | 9.4251 mL | 11.7813 mL |
| 10 mM | 0.2356 mL | 1.1781 mL | 2.3563 mL | 4.7125 mL | 5.8907 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4713 mL | 0.9425 mL | 1.1781 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4713 mL | 0.5891 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-O-Methylisoliquiritigenin
Catalog No.:BCX2091
CAS No.:476487-22-6
- Albanin D
Catalog No.:BCX2090
CAS No.:134955-26-3
- Notoginsenoside SFt4
Catalog No.:BCX2089
CAS No.:1351360-30-9
- Suksdorfin
Catalog No.:BCX2088
CAS No.:53023-17-9
- Rhamnetin 3-sophoroside
Catalog No.:BCX2087
CAS No.:259234-17-8
- Rhamnetin 3-O-gentiobioside
Catalog No.:BCX2086
CAS No.:1786990-15-8
- Methyl-6-gingerol
Catalog No.:BCX2085
CAS No.:23513-10-2
- 16-Acetoxy-7alpha-methoxyroyleanone
Catalog No.:BCX2084
CAS No.:109974-33-6
- Fischeroside A
Catalog No.:BCX2083
CAS No.:1307257-07-3
- 2-Hydroxy-6-methoxy-4-O-(6''-O-alpha-L-arabinofuranosyl-beta-D-glucopyranosyl)acetophenone
Catalog No.:BCX2082
CAS No.:1452160-87-0
- Isobiflorin
Catalog No.:BCX2081
CAS No.:152041-16-2
- Bracteanolide A
Catalog No.:BCX2080
CAS No.:1021432-39-2
- 5'-Methoxyoctahydrocurcumin
Catalog No.:BCX2093
CAS No.:718638-77-8
- 6-Hydroxykaempferol 7-glucoside
Catalog No.:BCX2094
CAS No.:70056-55-2
- Isosyringalide 3'-rhamnoside
Catalog No.:BCX2095
CAS No.:110326-99-3
- Tupichinol B
Catalog No.:BCX2096
CAS No.:497142-89-9
- Isorutarin
Catalog No.:BCX2097
CAS No.:53846-51-8
- Wattigenin A
Catalog No.:BCX2098
CAS No.:171485-66-8
- 4-(3,5-Dihydroxy-7-(4-hydroxy-3-methoxyphenyl)heptyl)benzene-1,2-diol
Catalog No.:BCX2099
CAS No.:884495-94-7
- 2-Methoxy-2-methyl-6-(4-methylphenyl)-4-heptanone
Catalog No.:BCX2100
CAS No.:70369-29-8
- Kaempferol 3-O-malonylglucoside
Catalog No.:BCX2101
CAS No.:81202-52-0
- 3',4'-Dimethoxytaxifolin
Catalog No.:BCX2102
CAS No.:179871-73-9
- Stellarin 2
Catalog No.:BCX2103
CAS No.:63975-58-6
- 25R-Tupistroside E
Catalog No.:BCX2104
CAS No.:1448639-95-9
Herbal Medicine and Voice Quality: Uncovering the Impact Through Acoustic Analysis.[Pubmed:39551657]
J Voice. 2024 Nov 16:S0892-1997(24)00377-1.
OBJECTIVE: Rutarin, an herbal formulation combining powdered seeds of Cydonia oblonga (quince) and aerial parts of Origanum majorana (marjoram), is used to address respiratory issues and enhance voice quality. This study investigates the effects of Rutarin on voice parameters, including fundamental frequency (F0), jitter, shimmer, harmonics-to-noise ratio (HNR), cepstral peak prominence (CPP), and smoothed cepstral peak prominence (CPPS). METHODS: Voice samples of 79 individuals who produced a sustained vowel /a/ were examined before and after consuming either Rutarin or warm water. The pretest vowel production was performed twice with a 5-minute interval between the first and second recordings before the actual test. Following consumption, the post test was performed five times for each participant at intervals immediately after drinking, 5, 15, 35, and 60 minutes. The repeated measure analysis of variance and Friedman test were employed to assess the within-subject differences, allowing the analysis of multiple conditions experienced by the same individual. RESULTS: Despite the recognized medicinal properties of its components, Rutarin did not produce significant improvements in voice quality compared to water. Water demonstrated notable effects on F0, jitter, HNR, CPP, and CPPS, particularly in male participants (P < 0.05). CONCLUSION: Although Rutarin may offer some therapeutic advantages for throat and respiratory health, it does not appear to enhance vocal performance as claimed.
Metabolome profile variations in common bean (Phaseolus vulgaris L.) resistant and susceptible genotypes incited by rust (Uromyces appendiculatus).[Pubmed:37007949]
Front Genet. 2023 Mar 16;14:1141201.
The causal agent of rust, Uromyces appendiculatus is a major constraint for common bean (Phaseolus vulgaris) production. This pathogen causes substantial yield losses in many common bean production areas worldwide. U. appendiculatus is widely distributed and although there have been numerous breakthroughs in breeding for resistance, its ability to mutate and evolve still poses a major threat to common bean production. An understanding of plant phytochemical properties can aid in accelerating breeding for rust resistance. In this study, metabolome profiles of two common bean genotypes Teebus-RR-1 (resistant) and Golden Gate Wax (susceptible) were investigated for their response to U. appendiculatus races (1 and 3) at 14- and 21-days post-infection (dpi) using liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (LC-qTOF-MS). Non-targeted data analysis revealed 71 known metabolites that were putatively annotated, and a total of 33 were statistically significant. Key metabolites including flavonoids, terpenoids, alkaloids and lipids were found to be incited by rust infections in both genotypes. Resistant genotype as compared to the susceptible genotype differentially enriched metabolites including aconifine, D-sucrose, galangin, Rutarin and others as a defence mechanism against the rust pathogen. The results suggest that timely response to pathogen attack by signalling the production of specific metabolites can be used as a strategy to understand plant defence. This is the first study to illustrate the utilization of metabolomics to understand the interaction of common bean with rust.
[Chemical constituents from roots of Peucedanum praeruptorum (V)].[Pubmed:23477142]
Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3573-6.
OBJECTIVE: To study the chemical constituents in roots of Peucedanum praeruptorum from Henan. METHOD: The constituents were isolated by column chromatography on silica gel and ODS, and identified by NMR, MS spectroscopic methods. RESULT: Six compounds, Pd-I b(1), marmesinsin (2), Rutarin (3), isoRutarin (4), 4H-1-benzopyran-4-one, 5-hydroxy-6-methoxy-2-phenyl-7-O-alpha-D-glucuronyl methyl ester (5), 4H-1-benzopyran-4-one, 5-hydroxy-6-methoxy-2-phenyl-7-O-alpha-D-glucuronyl acid (6) were isolated and identified. CONCLUSION: Compound 5 was a new compound, compounds 5 and 6 were isolated from Peucedanum plant for the first time.
Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed:21985060]
J Nat Prod. 2011 Oct 28;74(10):2286-9.
From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark. Thus, from the cyclohexane, ethyl acetate, and methanol extracts, two new heterocyclic compounds, omubioside (1) and katimborine (2), were isolated in addition to five known coumarins (Rutarin (3), seselin (4), suberosin (5), demethylsuberosin (6), and haploperoside (7)), two known alkaloids (5-hydroxynoracronycine (8) and 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (9)), trigonelline (10), and the limonoid 7alpha-obacunyl acetate (11). The best growth inhibitors of Plasmodium falciparum were alkaloids 8 and 9, with IC50 values of 0.9 and 3.0 mug/mL.
Structures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu.[Pubmed:17262257]
Planta Med. 1989 Feb;55(1):64-7.
From the n-butanol extract of the crude drug "Bai-Hua Qian-Hu", the root of PEUCEDANUM PRAERUPTORUM Dunn. (Umbelliferae) praeroside I ( 1) and three known linear-type furanocoumarin glycosides, isoRutarin ( 2), Rutarin ( 3), and marmesinin ( 4), along with two known simple coumarin glycosides, scopolin ( 5) and skimmin ( 6), were isolated. The structure of praeroside I was established as rutaretin-4'- O-(6-vanilloyl-beta- D-glucopyranoside) by spectroscopic and chemical methods.


