Kushenol CCAS# 99119-73-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99119-73-0 | SDF | Download SDF |
| PubChem ID | 5481237 | Appearance | Yellow powder |
| Formula | C25H26O7 | M.Wt | 438.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
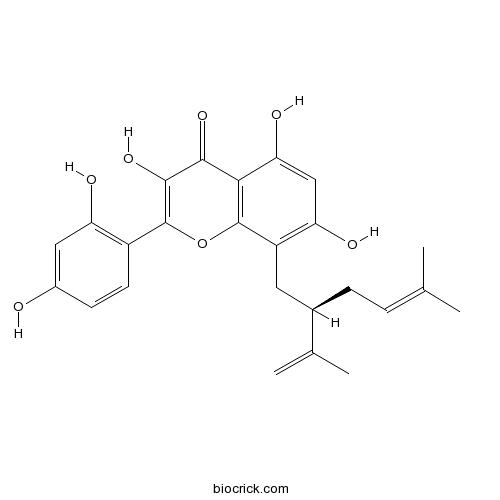
| Chemical Name | 2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-8-[(2R)-5-methyl-2-prop-1-en-2-ylhex-4-enyl]chromen-4-one | ||
| SMILES | CC(=CCC(CC1=C(C=C(C2=C1OC(=C(C2=O)O)C3=C(C=C(C=C3)O)O)O)O)C(=C)C)C | ||
| Standard InChIKey | WAAPHYJTKSTXSX-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C25H26O7/c1-12(2)5-6-14(13(3)4)9-17-19(28)11-20(29)21-22(30)23(31)25(32-24(17)21)16-8-7-15(26)10-18(16)27/h5,7-8,10-11,14,26-29,31H,3,6,9H2,1-2,4H3/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Kushenol C is a good 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenger, and it exhibits inhibitory activity against Sodium-dependent glucose cotransporter 2(SGLT2). Kushenol C shows antimicrobial activity against Staphylococcus aureus and Streptococcus mutans. Kushenol C may be potent preventive and therapeutic candidates for Alzheimer's disease, it (IC(50) 5.45 microM) can inhibit beta-site APP cleaving enzyme 1 (BACE1) activities. |
| Targets | ROS | BACE1 | Antifection | SGLT2 | NF-κB |
| In vitro | Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens.[Pubmed: 18451517]Biol. Pharm. Bull., 2008, 31(5):908-15.
|
| Kinase Assay | Selective inhibition of prenylated flavonoids from Sophora flavescens against BACE1 and cholinesterases.[Pubmed: 20387235 ]Am. J. Chinese Med., 2012, 38(2):415-29.It was previously reported that certain lavandulylated flavanones from Sophora flavescens are beta-site APP cleaving enzyme 1 (BACE1) inhibitors; however, based upon their levels within the extract, their inhibitory effects should be higher than expected. Moreover, chalcones and flavonols were reported to exert higher bioactivities than flavanones. These findings have led to a further search for other possible constituents potentially contributing to the strong inhibitory activity of the S. flavescens extract. |

Kushenol C Dilution Calculator

Kushenol C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2805 mL | 11.4025 mL | 22.805 mL | 45.61 mL | 57.0125 mL |
| 5 mM | 0.4561 mL | 2.2805 mL | 4.561 mL | 9.122 mL | 11.4025 mL |
| 10 mM | 0.2281 mL | 1.1403 mL | 2.2805 mL | 4.561 mL | 5.7013 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4561 mL | 0.5701 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kushenol E
Catalog No.:BCN3348
CAS No.:99119-72-9
- Kushenol I
Catalog No.:BCN2983
CAS No.:99119-69-4
- Limonexic acid
Catalog No.:BCN4534
CAS No.:99026-99-0
- [Ala113]-MBP (104-118)
Catalog No.:BCC5836
CAS No.:99026-78-5
- [Ala107]-MBP (104-118)
Catalog No.:BCC5835
CAS No.:99026-77-4
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
- Imiquimod
Catalog No.:BCC2492
CAS No.:99011-02-6
- Fentanyl citrate
Catalog No.:BCC6000
CAS No.:990-73-8
- 4-Hydroxybenzoic acid
Catalog No.:BCN4546
CAS No.:99-96-7
- 4'-Hydroxyacetophenone
Catalog No.:BCN4544
CAS No.:99-93-4
- 4-Isopropyltoluene
Catalog No.:BCC8282
CAS No.:99-87-6
- Methyl 4-hydroxybenzoate
Catalog No.:BCN4540
CAS No.:99-76-3
- Yadanzioside I
Catalog No.:BCN6715
CAS No.:99132-95-3
- Yadanzioside L
Catalog No.:BCN6713
CAS No.:99132-97-5
- Dehydrobruceantinol
Catalog No.:BCN7621
CAS No.:99132-99-7
- 1,6,8-Trideoxyshanzhigenin
Catalog No.:BCN6909
CAS No.:99173-00-9
- Salannin
Catalog No.:BCN8052
CAS No.:992-20-1
- Anpirtoline hydrochloride
Catalog No.:BCC6754
CAS No.:99201-87-3
- Kushenol A
Catalog No.:BCN2982
CAS No.:99217-63-7
- Kushenol B
Catalog No.:BCN3313
CAS No.:99217-64-8
- Caesalpin J
Catalog No.:BCC8305
CAS No.:99217-67-1
- Broussoflavonol B
Catalog No.:BCN3679
CAS No.:99217-70-6
- Mulberrofuran G pentaacetate
Catalog No.:BCN6518
CAS No.:99217-75-1
- Proglumide sodium salt
Catalog No.:BCC5768
CAS No.:99247-33-3
Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens.[Pubmed:18451517]
Biol Pharm Bull. 2008 May;31(5):908-15.
The objective of this research was to re-evaluate the antioxidant effects of the prenylated flavonoids from Sophora flavescens via in vitro 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), peroxynitrite (ONOO(-)), and total reactive oxygen species (ROS) assays. In addition, a further examination of kuraridinol, kurarinol, and kurarinone, also isolated from S. flavescens, was carried out by the inhibition of tert-butylhydroperoxide (t-BHP)-induced intracellular ROS generation and t-BHP-induced activation of nuclear factor-kappaB (NF-kappaB). Upon re-examination of the ethyl acetate (EtOAc) soluble fraction of S. flavescens, two major prenylated chalcones, including kuraridin and kuraridinol, along with a minor prenylated flavonol, Kushenol C, were isolated as good DPPH scavengers. This was in contrast to the prenylated flavanones, sophoraflavanone G and kurarinone, which were isolated from the methylene chloride (CH(2)Cl(2)) fraction of the same source. Five flavanones consisting of kushenol E, leachianone G, kurarinol, sophoraflavanone G, and kurarinone exhibited significant antioxidant potentials in the ABTS, ONOO(-), and total ROS assays; however, the prenylated chalcones and prenylated flavonol showed more potent scavenging/inhibitory activities than the prenylated flavanones. Therefore, the prenylated chalcones and prenylated flavonol, rather than the prenylated flavanones, may make important contributions toward the marked antioxidant capacities of S. flavescens. Furthermore, kuraridinol, kurarinol, and kurarinone showed significant inhibitory activities against intracellular ROS levels as well as NF-kappaB activation by t-BHP. Overall, the results indicate that S. flavescens and its prenylated flavonoids may possess good anti-inflammatory activity, which is implicated in their significant antioxidant activity.
Selective inhibition of prenylated flavonoids from Sophora flavescens against BACE1 and cholinesterases.[Pubmed:20387235]
Am J Chin Med. 2010;38(2):415-29.
It was previously reported that certain lavandulylated flavanones from Sophora flavescens are beta-site APP cleaving enzyme 1 (BACE1) inhibitors; however, based upon their levels within the extract, their inhibitory effects should be higher than expected. Moreover, chalcones and flavonols were reported to exert higher bioactivities than flavanones. These findings have led to a further search for other possible constituents potentially contributing to the strong inhibitory activity of the S. flavescens extract. In this study, BACE1 activities were significantly inhibited by 8-lavandulylkaempferol (IC(50) 7.29 microM), kuraridinol (IC(50) 7.10 microM), kuraridin (IC(50) 6.03 microM), and Kushenol C (IC(50) 5.45 microM) from the ethyl acetate fraction, along with desmethylanhydroicaritin (IC(50) 1.86 microM), xanthohumol (IC(50) 7.19 microM), and leachianone G (IC(50) 8.56 microM) from the dichloromethane fraction of the extract. The results indicate that the prenyl group, rather than the lavandulyl group, and the flavonols and chalcones, rather than flavanones, might make predominant contributions to BACE1 inhibition. In particular, 8-lavandulylkaempferol exhibited significant inhibitory effects with IC(50) values of 7.10 and 8.11 microM for butyrylcholinesterase and acetylcholinesterase, respectively, when compared to its counterpart, desmethylanhydroicaritin. This indicates that the lavandulyl group might play a predominant role in both cholinesterase inhibitions. This is the first study indicating that prenylated flavonoids exert varying degrees of inhibition primarily through their skeleton (flavonols, chalcones, flavanones), as well as their lipophilic chain length (prenyl and lavandulyl groups). Therefore, S. flavescens and its prenylated flavonoids, possessing low molecular weights and lipophilic moieties may be potent preventive and therapeutic candidates for Alzheimer's disease.


