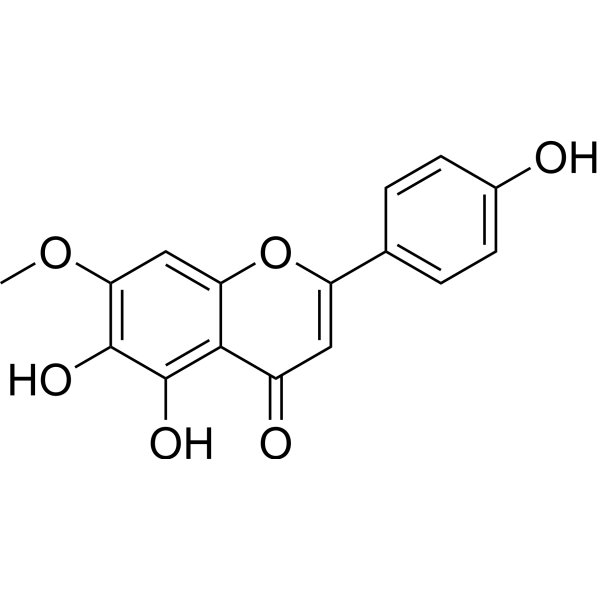
LadanetinCAS# 23130-22-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 23130-22-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C16H12O6 | M.Wt | 300.26 |
| Type of Compound | Flavones/Flavanones | Storage | Desiccate at -20°C |
| Synonyms | 7-O-Methylscutellarein,5,6,4\'-Trihydroxy-7-methoxyflavone,7-Methoxy-5,6,4\'-trihydroxyflavone,Sorbifo... | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ladanetin Dilution Calculator

Ladanetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3304 mL | 16.6522 mL | 33.3045 mL | 66.6089 mL | 83.2612 mL |
| 5 mM | 0.6661 mL | 3.3304 mL | 6.6609 mL | 13.3218 mL | 16.6522 mL |
| 10 mM | 0.333 mL | 1.6652 mL | 3.3304 mL | 6.6609 mL | 8.3261 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.6661 mL | 1.3322 mL | 1.6652 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.6661 mL | 0.8326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salvifaricin
Catalog No.:BCX1752
CAS No.:87321-87-7
- (5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-...
Catalog No.:BCX1751
CAS No.:121795-56-0
- (-)-3,3'-Bisdemethylpinoresinol
Catalog No.:BCX1750
CAS No.:340167-81-9
- Chavicol 1-O-β-D-glucopyranoside
Catalog No.:BCX1749
CAS No.:64703-98-6
- Tanegoside A
Catalog No.:BCX1748
CAS No.:131653-21-9
- Oxyresveratrol 4'-O-β-D-glucopyranoside
Catalog No.:BCX1747
CAS No.:863329-68-4
- Peucedanol
Catalog No.:BCX1746
CAS No.:46992-81-8
- Qianhucoumarin D
Catalog No.:BCX1745
CAS No.:20516-19-2
- Ophiopogonin R
Catalog No.:BCX1744
CAS No.:1418183-25-1
- Nortrachelogenin 4'-O-β-gentiobioside
Catalog No.:BCX1743
CAS No.:1961246-09-2
- (8R,8'R)-Matairesinol 4,4'-di-O-β-D-glucopyranoside
Catalog No.:BCX1742
CAS No.:41948-08-7
- Propionic acid (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b:3,4- b′]dipyran-2-one-...
Catalog No.:BCX1741
CAS No.:440094-34-8
- Pseudobatigenin-7-O-β-D-xylopyranosyl(1→6)-β-D-glucopyranosid
Catalog No.:BCX1754
CAS No.:224957-15-7
- Cnidioside B
Catalog No.:BCX1755
CAS No.:141896-54-0
- Inflexuside A
Catalog No.:BCX1756
CAS No.:1395048-85-7
- Bulnesol
Catalog No.:BCX1757
CAS No.:22451-73-6
- Glaucocalyxin D
Catalog No.:BCX1758
CAS No.:140671-02-9
- Salviandulin E
Catalog No.:BCX1759
CAS No.:158994-32-2
- Quercetin-3-O-(2''-O-galloyl)-β-D-glucopyranoside
Catalog No.:BCX1760
CAS No.:69624-79-9
- Inflexuside B
Catalog No.:BCX1761
CAS No.:1395048-86-8
- Irisquinone
Catalog No.:BCX1762
CAS No.:56495-82-0
- 2-Methoxy-6-pentadecyl-1,4-benzoquinone
Catalog No.:BCX1763
CAS No.:144078-11-5
- 2,4-Dihydroxy-allylbenzene-2-O-β-D-glucopyranoside
Catalog No.:BCX1764
CAS No.:1416564-61-8
- Polyfuroside
Catalog No.:BCX1765
CAS No.:108886-03-9
New isoflavones from Gynandriris sisyrinchium and their antioxidant and cytotoxic activities.[Pubmed:26410237]
Fitoterapia. 2015 Dec;107:15-21.
Chemical investigation of Gynandriris sisyrinchium (L.) Parl growing in Jordan resulted in the isolation and characterization of a total of twelve compounds two of which are reported here for the first time in nature. These new compounds included the isoflavones; 3'-methyl tenuifone (2) and gynandrinone (5). In addition, ten known compounds including; beta-sitosterol (1), 7,3'-dimethoxy-5,6,4'-trihydroxyisoflavone (3), iristectorigenin (4), hispidulin (6), galangustin (7), 6-hydroxybiochanin A (8), ursolic acid (9), Ladanetin (10), 4'-O-methylgenistein (11) and beta-sitosterol glucoside (12) are also reported here for the first time from G. sisyrinchium. The isolated compounds were characterized by different spectroscopic methods including NMR (1D and 2D), UV, IR and MS (HRESIMS and EIMS). The antioxidant and cytotoxic activities of isoflavones 2, 3 and 5 were investigated. Compound 3 showed the highest antioxidant activity (IC50=17.3mug/mL), as compared to compounds 5 and 2 (IC50=26.7 and 51.7mug/mL, respectively). The cytotoxic activity against the human promyelocytic leukemia HL-60 cells revealed that compound 2 was the most active (40muM). The results indicate that the cytotoxicity of compound 2 is mediated by apoptosis.
Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells.[Pubmed:20932762]
Bioorg Med Chem Lett. 2010 Nov 15;20(22):6411-5.
Two new flavonoids, Ladanetin-6-O-beta-(6''-O-acetyl)glucoside (1) and pedalitin-3'-O-beta-glucoside (2), together with 15 known compounds (3-17), were isolated from the whole plants of Dracocephalum tanguticum. Their structures were established on the basis of extensive spectroscopic (IR, MS, 2D NMR) data analysis and by the comparison with spectroscopic data reported in the literature. Antioxidant capacities of the isolated substances were determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferrous ions, and ABTS(.)(+) radical in vitro assay, and their cytoprotective activities were also tested on doxorubicin (DOX)-induced toxicity in H9c2 cardiomyocytes. Among all the tested compounds, luteolin-7-O-beta-D-glucopyranoside (7) exhibited both strong antioxidative effect and high protective activity against DOX-induced toxicity. Further investigation found 7 could decrease DOX-induced death of H9c2 cell, reduce LDH and CK level, and inhibit the elevated intracellular concentration of ROS and [Ca(2+)](i). The preliminary structure-activity relationships (SAR) of these compounds revealed the Delta(2,3) double bond on C-ring and 3',4'-di-OHs on B-ring with a flavone skeleton such as luteolin and its derivatives, were necessary for their cardioprotective effects.
Flavones and flavone glycosides from Halophila johnsonii.[Pubmed:18771781]
Phytochemistry. 2008 Oct;69(14):2603-8.
Halophila johnsonii Eiseman is a shallow-water marine angiosperm which contains UV-absorbing metabolites. Studies on methanol extracts of H. johnsonii by means of HPLC-UV, NMR, HPLC-MS resulted in isolation and identification of seven previously unknown flavone glycosides: 5,6,7,3',4',5'-hexahydroxyflavone-7-O-beta-glucopyranoside (1), 5,6,7,3',4',5'-hexahydroxyflavone-7-O-(6''-O-acetyl)-beta-glucopyranoside (2), 6-hydroxyluteolin-7-O-(6''-O-acetyl)-beta-glucopyranoside (3), 6-hydroxyapigenin-7-O-(6''-O-acetyl)-beta-glucopyranoside (4), 6-hydroxyapigenin-7-O-(6''-O-[E]-coumaroyl)-beta-glucopyranoside (5), 6-hydroxyapigenin-7-O-(6''-O-[E]-caffeoyl)-beta-glucopyranoside (6) and 6-hydroxyluteolin-7-O-(6''-O-[E]-coumaroyl)-beta-glucopyranoside (7). Also isolated were three known flavone glycosides, 6-hydroxyluteolin 7-O-beta-glucopyranoside (8), scutellarein-7-O-beta-glucopyranoside (9), and spicoside (10), and five known flavones, pedalitin (11), Ladanetin (12), luteolin (13), apegenin (14) and myricetin (15). Qualitative comparison of the flavonoid distribution in the leaf and rhizome-root portions of the plant was also investigated, with the aim of establishing the UV-protecting roles that flavonoids played in the sea grass.
[Flavones from flowers of Sesamum indicum].[Pubmed:17583201]
Zhongguo Zhong Yao Za Zhi. 2007 Apr;32(7):603-5.
OBJECTIVE: To investigate the chemical constituents from flowers of Sesamum indicum. METHOD: Column chromatography with silica gel, C18 and Sephadex LH -20 as packing materials was used to separate the chemical constituents, and the structures were determined by chemical and spectroscopic methods. RESULT: Six flavones were isolated and elucidated as apigenin (1), Ladanetin (2), Ladanetin-6-O-beta-D-glucoside (3), apigenin-7-O-glucuronic acid (4), pedalitin (5), and pedalitin-6-O-glucoside (6). CONCLUSION: All of the compounds were isolated from this plant for the first time.


