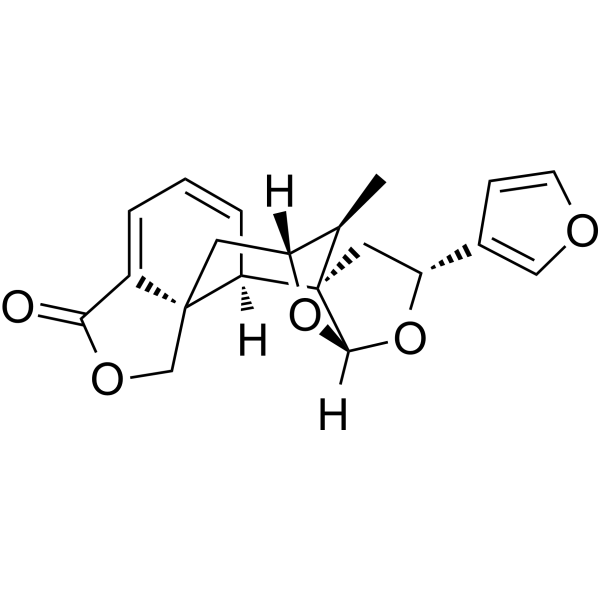
SalvifaricinCAS# 87321-87-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 87321-87-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H20O5 | M.Wt | 340.37 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Salvifaricin Dilution Calculator

Salvifaricin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.938 mL | 14.6899 mL | 29.3798 mL | 58.7596 mL | 73.4495 mL |
| 5 mM | 0.5876 mL | 2.938 mL | 5.876 mL | 11.7519 mL | 14.6899 mL |
| 10 mM | 0.2938 mL | 1.469 mL | 2.938 mL | 5.876 mL | 7.3449 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5876 mL | 1.1752 mL | 1.469 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5876 mL | 0.7345 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-...
Catalog No.:BCX1751
CAS No.:121795-56-0
- (-)-3,3'-Bisdemethylpinoresinol
Catalog No.:BCX1750
CAS No.:340167-81-9
- Chavicol 1-O-β-D-glucopyranoside
Catalog No.:BCX1749
CAS No.:64703-98-6
- Tanegoside A
Catalog No.:BCX1748
CAS No.:131653-21-9
- Oxyresveratrol 4'-O-β-D-glucopyranoside
Catalog No.:BCX1747
CAS No.:863329-68-4
- Peucedanol
Catalog No.:BCX1746
CAS No.:46992-81-8
- Qianhucoumarin D
Catalog No.:BCX1745
CAS No.:20516-19-2
- Ophiopogonin R
Catalog No.:BCX1744
CAS No.:1418183-25-1
- Nortrachelogenin 4'-O-β-gentiobioside
Catalog No.:BCX1743
CAS No.:1961246-09-2
- (8R,8'R)-Matairesinol 4,4'-di-O-β-D-glucopyranoside
Catalog No.:BCX1742
CAS No.:41948-08-7
- Propionic acid (9R,10R)-9-acetoxy-8,8-dimethyl-9,10-dihydro-2H,8H-benzo[1,2-b:3,4- b′]dipyran-2-one-...
Catalog No.:BCX1741
CAS No.:440094-34-8
- 1α-Hydroxy-3-deoxypseudoanisatin
Catalog No.:BCX1740
CAS No.:258854-65-8
- Ladanetin
Catalog No.:BCX1753
CAS No.:23130-22-5
- Pseudobatigenin-7-O-β-D-xylopyranosyl(1→6)-β-D-glucopyranosid
Catalog No.:BCX1754
CAS No.:224957-15-7
- Cnidioside B
Catalog No.:BCX1755
CAS No.:141896-54-0
- Inflexuside A
Catalog No.:BCX1756
CAS No.:1395048-85-7
- Bulnesol
Catalog No.:BCX1757
CAS No.:22451-73-6
- Glaucocalyxin D
Catalog No.:BCX1758
CAS No.:140671-02-9
- Salviandulin E
Catalog No.:BCX1759
CAS No.:158994-32-2
- Quercetin-3-O-(2''-O-galloyl)-β-D-glucopyranoside
Catalog No.:BCX1760
CAS No.:69624-79-9
- Inflexuside B
Catalog No.:BCX1761
CAS No.:1395048-86-8
- Irisquinone
Catalog No.:BCX1762
CAS No.:56495-82-0
- 2-Methoxy-6-pentadecyl-1,4-benzoquinone
Catalog No.:BCX1763
CAS No.:144078-11-5
- 2,4-Dihydroxy-allylbenzene-2-O-β-D-glucopyranoside
Catalog No.:BCX1764
CAS No.:1416564-61-8
Neo-clerodane Diterpenoids with Hypoglycemic Effects in Vivo from the Aerial Parts of Salvia hispanica L.[Pubmed:34292661]
Chem Biodivers. 2021 Sep;18(9):e2100517.
A new neo-clerodane diterpenoid, salvihispin H (1), and six known ones (2-7) were identified from the aerial parts of Salvia hispanica L. The structure and absolute configuration of 1 were elucidated by extensive analysis of spectroscopic ((1) H, (13) C, and 2D NMR, and HR-ESI-MS) and single-crystal X-ray diffraction data. The anti-diabetic effects of salvihispin H (1) and Salvifaricin (2) were evaluated in diabetic db/db mice. The data showed that 1 and 2 could significantly reduce fasting blood glucose level and improve insulin resistance, and compound 1 exerted glucose-lowering effect more quickly than metformin. In addition, 1 and 2 could also reduce serum TG level in db/db mice. These results demonstrated that compounds 1 and 2 could be considered as potent candidates for the therapy of type 2 diabetes mellitus (T2DM).
Isolation and chemical modification of clerodane diterpenoids from Salvia species as potential agonists at the kappa-opioid receptor.[Pubmed:17638340]
Chem Biodivers. 2007 Jul;4(7):1586-93.
The clerodane diterpenoid salvinorin A (1), the main active component of the psychotropic herb Salvia divinorum, has been reported to be a potent agonist at the kappa-opioid receptor. Computer modeling suggested that splendidin (2) from S. splendens, as well as related compounds, might possess similar activities. In the present study, this hypothesis was tested by determination of the binding properties of a series of structural congeners, compounds 2-8, at the mu-, delta-, and kappa-opioid receptors. However, none of these compounds showed significant binding to any of the opioid-receptor subtypes, thus disproving the above hypothesis. The novel compounds 7 and 8 were obtained semi-synthetically by selective modification of salvifarin (5), isolated from Salvia farinacea, upon epoxide-ring opening with AcOH in the presence of indium(III) triflate. Also, the X-ray crystal structure of Salvifaricin (6; Fig.), obtained from S. farinacea, was determined for the first time and used, in combination with in-depth NMR experiments, to elucidate the absolute configurations of the new products. Our experiments demonstrate that the relatively well-accessible diterpenoid 6 could be used as starting material for future studies into the structure-activity relationship at the kappa-opioid receptor.
A novel diterpenoid with a rearranged neoclerodane skeleton from Salvia leucantha CAV.[Pubmed:29435878]
J Nat Med. 2006 Jul;60(3):206-209.
A new diterpenoid with a rearranged neoclerodane skeleton, spiroleucantholide (compound "S1"), along with four known diterpenoids-Salvifaricin (compound "S2"), isosalvipuberulin (compound "S3"), salvileucantholide (compound "S4"), and salviandulin E (compound "S5")-was isolated from the aerial parts of Salvia leucantha CAV.. The structures were established by spectroscopic methods, including the X-ray analysis of spiroleucantholide (S1).
Polystachynes A-E, five cis-neo-clerodane diterpenoids from Salvia polystachya.[Pubmed:10656416]
Phytochemistry. 2000 Jan;53(1):103-9.
From the aerial parts of Salvia polystachya five new neo-clerodane diterpenoids, polystachynes A-E, have been isolated. The structures were established by spectroscopic methods, including the X-ray analysis of polystachynes C and D. The known clerodanes Salvifaricin, linearolactone and dehydrokerlin were also isolated.


