Levulinic acidCAS# 123-76-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123-76-2 | SDF | Download SDF |
| PubChem ID | 11579 | Appearance | Solid |
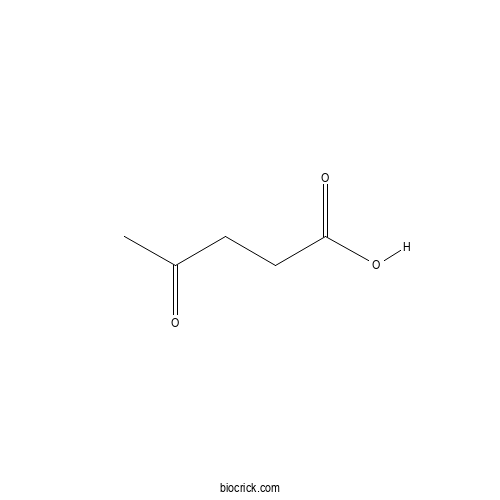
| Formula | C5H8O3 | M.Wt | 116.11 |
| Type of Compound | Other NPs | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-oxopentanoic acid | ||
| SMILES | CC(=O)CCC(=O)O | ||
| Standard InChIKey | JOOXCMJARBKPKM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H8O3/c1-4(6)2-3-5(7)8/h2-3H2,1H3,(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Levulinic acid Dilution Calculator

Levulinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6125 mL | 43.0626 mL | 86.1252 mL | 172.2505 mL | 215.3131 mL |
| 5 mM | 1.7225 mL | 8.6125 mL | 17.225 mL | 34.4501 mL | 43.0626 mL |
| 10 mM | 0.8613 mL | 4.3063 mL | 8.6125 mL | 17.225 mL | 21.5313 mL |
| 50 mM | 0.1723 mL | 0.8613 mL | 1.7225 mL | 3.445 mL | 4.3063 mL |
| 100 mM | 0.0861 mL | 0.4306 mL | 0.8613 mL | 1.7225 mL | 2.1531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4''-O-Glucosyl-17α-deacetyltanghinin
Catalog No.:BCX0394
CAS No.:114612-75-8
- Tetrahydrorhombifoline
Catalog No.:BCX0393
CAS No.:3382-84-1
- n-Butyl α-D-fructofuranoside
Catalog No.:BCX0392
CAS No.:80971-59-1
- Excoecafolin C
Catalog No.:BCX0391
CAS No.:1643370-00-6
- (2E,6E)-Farnesyl acetate
Catalog No.:BCX0390
CAS No.:4128-17-0
- 4,4'-(1,3-Dimethylbutylidene)diphenol
Catalog No.:BCX0389
CAS No.:6807-17-6
- 14,15,16-Trinorlabda-8(17),11-dien-13-oic acid
Catalog No.:BCX0388
CAS No.:917078-12-7
- Sclerone
Catalog No.:BCX0387
CAS No.:19638-58-5
- Malformin C
Catalog No.:BCX0386
CAS No.:59926-78-2
- Gnetuhainin I
Catalog No.:BCX0385
CAS No.:308105-06-8
- Asperazine
Catalog No.:BCX0384
CAS No.:198953-76-3
- Triptotriterpenic acid C
Catalog No.:BCX0383
CAS No.:123914-32-9
- 17α-Deacetyltanghinin
Catalog No.:BCX0396
CAS No.:111614-46-1
- 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate
Catalog No.:BCX0397
CAS No.:148757-94-2
- Disporopsin
Catalog No.:BCX0398
CAS No.:1430334-05-6
- 8-Methyldisporopsin
Catalog No.:BCX0399
CAS No.:1671098-51-3
- 8-Methyldisporopsin 4'-methyl ether
Catalog No.:BCX0400
CAS No.:1589545-88-9
- 8-Methyl-2'-deoxydisporopsin
Catalog No.:BCX0401
CAS No.:1671098-52-4
- Disporopsin 4'-methyl ether
Catalog No.:BCX0402
CAS No.:1671098-53-5
- Ichanexic acid
Catalog No.:BCX0403
CAS No.:1044818-57-6
- 3''-Methoxycentrolobol
Catalog No.:BCX0404
CAS No.:811471-19-9
- Kaempferol 7-O-(4''-O-methyl)glucoside
Catalog No.:BCX0405
CAS No.:1092795-48-6
- Suberanilic acid
Catalog No.:BCX0406
CAS No.:149648-52-2
- N1-Phenylsuberamide
Catalog No.:BCX0407
CAS No.:1305124-48-4
Enzymatic synthesis of a novel solid-liquid phase change energy storage material based on levulinic acid and 1,4-butanediol.[Pubmed:38647853]
Bioresour Bioprocess. 2022 Feb 11;9(1):12.
The current energy crisis has prompted the development and utilization of renewable energy and energy storage material. In this study, Levulinic acid (LA) and 1,4-butanediol (BDO) were used to synthesize a novel Levulinic acid 1,4-butanediol ester (LBE) by both enzymatic and chemical methods. The enzymatic method exhibited excellent performance during the synthesis process, and resulted in 87.33% of LBE yield, while the chemical method caused more by-products and higher energy consumption. What's more, the thermal properties of the obtained LBE as a phase change material (PCM) were evaluated. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) showed that the melting temperature, latent heat of melting, and pyrolysis temperature were 50.51 degrees C, 156.1 J/g, and 150-160 degrees C, respectively. Compared with the traditional paraffin, the prepared PCM has a superior phase transition temperature, a higher latent heat of melting, and better thermal stability. The thermal conductivity could be increased to 0.34 W/m/k after adding expanded graphite (EG). In summary, LBE has great potential in the application of energy storage as a low-temperature phase change energy storage material.
Construction of fluorescent sensor array with nitrogen-doped carbon dots for sensing Sudan Orange G and identification of various azo compounds.[Pubmed:38640659]
J Colloid Interface Sci. 2024 Apr 16;667:403-413.
In this study, nitrogen-doped carbon dots (N-CDs) were facilely fabricated by one-pot hydrothermal method with Levulinic acid and triethanolamine. A fluorescent sensor array was established for identifying azo compounds including Sudan Orange G (SOG), p-diaminoazobenzene, p-aminoazobenzene, azobenzene and quantitative detection of SOG. Experimental results revealed that azo compounds could quench the fluorescent intensity of N-CDs. Owing to various azo compounds showing different affinities to N-CDs, the sensor array exhibited different fluorescence quenching changes, which were further analyzed with principal component analysis to discriminate azo compounds. The sensor array was able to differentiate and recognize diverse concentrations of azo compounds from 0.25 to 2 mg/L. Simultaneously, a variety of factors affecting the detection of SOG were optimized. Under the optimized conditions, the sensor showed excellent stability and sensitivity. The sensor possessed marvelous linearity in the range of 0.1-1 mg/L and 1-4 mg/L and the detection limit was 27.82 mug/L. Spiked recoveries of 90.8-98.2 % were attained at spiked levels of 0.2 mg/L and 1 mg/L, demonstrating that the constructed fluorescence sensor was dependable and feasible for sensing SOG in environmental water samples.
Arginine Acts as both Co-Solvent and Catalyst in Regioselective Eutectic-Mediated Dimerization of Levulinic Acid.[Pubmed:38575387]
ChemSusChem. 2024 Apr 4:e202400503.
A simple, solvent-free arginine-catalyzed aldol dimerization of Levulinic acid was achieved via the simultaneous formation of a eutectic mixture. Dimers of Levulinic acid are valued as biomass-derived fine chemical precursors, with potential to upgrade to bio-jet fuels or N-containing functional chemicals. Typically, these dimers are produced as isomeric mixtures using high temperatures and a variety of solid inorganic catalysts or mineral acids. In this study, an organocatalytic and regioselective dimerization was achieved at 22% conversion on either a bench or kilogram scale using mild temperatures and only L-arginine as both a co-solvent and catalyst. The intricate H-bonding network comprising the eutectic solvent was harnessed to produce only one product, minimizing side reactivity and preserving the reactants for recycling.
Evaluation of the potential of Delta-aminolevulinic acid for simultaneous detection of bioburden and anti-microbial photodynamic therapy of MRSA infected wounds in Swiss albino mice.[Pubmed:38513542]
J Photochem Photobiol B. 2024 May;254:112892.
BACKGROUND: The dramatic increase of drug-resistant bacteria necessitates urgent development of platforms to simultaneously detect and inactivate bacteria causing wound infections, but are confronted with various challenges. Delta amino Levulinic acid (ALA) induced protoporphyrin IX (PpIX) can be a promising modality for simultaneous bioburden diagnostics and therapeutics. Herein, we report utility of ALA induced protoporphyrin (PpIX) based simultaneous bioburden detection, photoinactivation and therapeutic outcome assessment in methicillin resistant Staphylococcus aureus (MRSA) infected wounds of mice. METHODS: MRSA infected wounds treated with 10% ALA were imaged with help of a blue LED ( approximately 405 nm) based, USB powered, hand held device integrated with a modular graphic user interface (GUI). Effect of ALA application time, bacteria load, post bacteria application time points on wound fluorescence studied. PpIX fluorescence observed after excitation with blue LEDs was used to detect bioburden, start red light mediated antimicrobial photodynamic therapy (aPDT), determine aPDT effectiveness and assess selectivity of the approach. RESULTS: ALA-PpIX fluorescence of wound bed discriminates infected from uninfected wounds and detects clinically relevant load. While wound fluorescence pattern changes as a function of ALA incubation and post infection time, intra-wound inhomogeneity in fluorescence correlates with the Gram staining data on presence of biofilms foci. Lack of red fluorescence from wound granulation tissue treated with ALA suggests selectivity of the approach. Further, significant reduction ( approximately 50%) in red fluorescence, quantified using the GUI, relates well with bacteria load reduction observed post topical aPDT. CONCLUSION: The potential of ALA induced PpIX for simultaneous detection of bioburden, photodynamic inactivation and "florescence-guided aPDT assessment" is demonstrated in MRSA infected wounds of mice.
Integrated preparation of functional lignin nanoparticles and levulinic acid directly from the pre-hydrolysis liquor of poplar wood.[Pubmed:38493611]
Int J Biol Macromol. 2024 Apr;265(Pt 1):130906.
The pre-hydrolysis liquor (PHL) produced during pulp dissolution and biomass refining is mainly composed of hemicellulose and lignin, and it is a potential source for production of value-added materials and platform chemicals; however, their utilization has been a serious challenge. In this study, we proposed a green and simple strategy to simultaneously prepare size-controlled functional lignin nanoparticles (LNPs) and Levulinic acid (LA) from PHL as the raw material. The as-prepared LNPs exhibited remarkable stability thanks to the presence of saccharides with abundant oxygen-containing groups and surface charges, which prevented aggregation and maintained long-term storage stability. Trace amounts of the LNPs (70 % after five cycles. Overall, this green and simple strategy effectively and comprehensively utilized the PHL and showed potential for producing biobased nanomaterials and platform chemicals.
Ferrocene-functionalized zirconium-oxo clusters for achieving high-performance thermocatalytic redox reactions.[Pubmed:38485624]
Sci Bull (Beijing). 2024 Feb 27:S2095-9273(24)00134-8.
The Zr(IV) ions are easily hydrolyzed to form oxides, which severely limits the discovery of new structures and applications of Zr-based compounds. In this work, three ferrocene (Fc)-functionalized Zr-oxo clusters (ZrOCs), Zr(9)Fc(6), Zr(10)Fc(6) and Zr(12)Fc(8) were synthesized through inhibiting the hydrolysis of Zr(IV) ions, which show increased nuclearity and regular structural variation. More importantly, these Fc-functionalized ZrOCs were used as heterogeneous catalysts for the transfer hydrogenation of Levulinic acid (LA) and phenol oxidation reactions for the first time, and displayed outstanding catalytic activity. In particular, Zr(12)Fc(8) with the largest number of Zr active sites and Fc groups can achieve > 95% yield for LA-to-gamma-valerolactone within 4 h (130 degrees C) and > 98% yield for 2,3,6-trimethylphenol-to-2,3,5-trimethyl-p-benzoquinone within 30 min (80 degrees C), showing the best catalytic performance. Catalytic characterization combined with theory calculations reveal that in the Fc-functionalized ZrOCs, the Zr active sites could serve as substrate adsorption sites, while the Fc groups could act as hydrogen transfer reagent or Fenton reagent, and thus achieve effectively intramolecular metal-ligand synergistic catalysis. This work develops functionalized ZrOCs as catalysts for thermal-triggered redox reactions.
Extraction of 5-Hydroxymethylfurfural and Furfural in Aqueous Biphasic Systems: A COSMO-RS Guided Approach to Greener Solvent Selection.[Pubmed:38456191]
ACS Sustain Chem Eng. 2024 Feb 20;12(9):3766-3779.
5-Hydroxymethylfurfural (HMF) and furfural (Fur) are promising biobased platform chemicals, derived from the dehydration of carbohydrate feedstocks, normally conducted in an aqueous phase. Plagued by side-reactions in such phase, such as the rehydration to Levulinic acid (LA) and formic acid (FA) or self-condensation to humins, HMF and Fur necessitates diversification from monophasic aqueous reaction systems toward biphasic systems to mitigate undesired side-reactions. Here, a methodology based on the COnductor-like Screening MOdel for Real Solvents (COSMO-RS) method was used to screen solvent candidates based on the predicted partition coefficients (K(i)). Hansen solubility parameters in conjunction with excess thermodynamic quantities determined by COSMO-RS were employed to assess solvent compatibility. Experimental validation of the COSMO-RS values highlighted only minor deviations from the predictions with root-mean-square-error (RMSE) values of HMF and Fur at 0.76 and 5.32, respectively, at 298 K. The combined effort suggested cyclohexanone, isophorone, and methyl isobutyl ketone (MIBK) as the best candidates. Finally, extraction solvent reuse demonstrated cyclohexanone suitability for HMF extraction with K(HMF) of 3.66 and MIBK for Fur with K(Fur) 7.80 with consistent partitioning across four total runs. Both solvents are classified as recommended by the CHEM21 solvent selection guide, hence adding to the sustainability of the process.
Synthesis and Properties of a Novel Levulinic Acid-Based Environmental Auxiliary Plasticizer for Poly(vinyl chloride).[Pubmed:38337249]
Polymers (Basel). 2024 Jan 29;16(3):361.
Herein, a bio-based plasticizer ketalized tung oil butyl levulinate (KTBL) was developed using methyl eleostearate, a derivative of tung oil, and butyl levulinate. KTBL can be used as an auxiliary plasticizer to partially replace traditional plasticizer. The plasticizer has a ketone structure, an ester base, and a long linear chain. It was mixed with dioctyl phthalate (DOP), and the effect of the plasticizer KTBL as an auxiliary plasticizer on the plasticization of poly(vinyl chloride) (PVC) was studied. Their compatibility and plasticizing effect were evaluated using dynamic-mechanical thermal analysis (DMA), mechanical property analysis, and thermogravimetric analysis (TGA). The results demonstrate that when the KTBL to DOP ratio is 1:1, the blended sample with KTBL exhibits superior mechanical performance compared to pure DOP, resulting in an increased elongation at break from 377.47% to 410.92%. Moreover, with the increase in KTBL content, the durability is also significantly improved. These findings suggest that KTBL can serve as an effective auxiliary plasticizer for PVC, thereby reducing the reliance on DOP.
The Effects of Nine Compounds on Aldehyde-Oxidase-Related Genes in Bactrocera dorsalis (Hendel).[Pubmed:38254925]
Genes (Basel). 2023 Dec 25;15(1):35.
Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) (B. dorsalis) is an important agricultural, major invasive, and quarantine pest that can cause significant damage to the economic value of the fruit and vegetable industry. Male bait is one of the most effective methods of surveying, monitoring, and controlling B. dorsalis. In our study, we constructed cDNA libraries using total RNA extracted independently from the antennae, mouthparts, and thoracic legs of male and female adults and the ovipositors of female adults and screened out four aldehyde-oxidase-related genes (AOX-related), C58800, C66700, C67485, and C67698. Molecular docking predictions showed that eight compounds, including 3,4-dimethoxycinnamyl alcohol, 3,4-dimethoxy-cinnamaldehyde, deet, ethyl N-acetyl-N-butyl-beta-alaninate, n-butyl butyrate, n-butyl butyrate, ethyl butyrate, methyl eugenol, and ethyl acetate, could combine with proteins encoded by the four B. dorsalis AOX-related genes. Furthermore, QPCR was performed to confirm that four compounds, including 3,4-dimethoxy cinnamic aldehyde, butyl Levulinic acid ethyl ester (mosquito repellent), butyl butyrate, and methyl eugenol, induced significant changes in the AOX-related genes of B. dorsalis. These results provide useful information and guidance for the batch screening of potentially useful compounds and the search for effective attractants of B. dorsalis.
Lactic Acid Salts of Nicotine Potentiate the Transfer of Toxic Metals into Electronic Cigarette Aerosols.[Pubmed:38251020]
Toxics. 2024 Jan 13;12(1):65.
The designs and liquid formulations of Electronic Nicotine Delivery System (ENDS) devices continue to rapidly evolve. Thus, it is important to monitor and characterize ENDS aerosols for changes in toxic constituents. Many ENDS liquid formulations now include the addition of organic acids in a 1 to 1 molar ratio with nicotine. Metal concentrations in aerosols produced by ENDS devices with different nicotine salt formulations were analyzed. Aerosols from devices containing lactic acid had higher nickel, zinc, copper, and chromium concentrations than aerosols produced by devices containing benzoic acid or Levulinic acid. Our scanning electron microscope with energy dispersive X-ray analytical findings showed that the metals determined in the inductively coupled plasma-mass spectrometry analytical results were consistent with the metal compositions of the ENDS device components that were exposed to the liquids and that nickel is a major constituent in many ENDS internal components. As a result of the exposure of the nickel-containing components to the ENDS liquids, resulting aerosol nickel concentrations per puff were higher from devices that contained lactic acid in comparison to devices with benzoic or Levulinic acid. The aerosol nickel concentrations in 10 puffs from ENDS-containing lactic acid were, in some cases, hundreds of times higher than cigarette mainstream smoke nickel deliveries. Thus, the design of an ENDS device in terms of both physical construction components and the liquid chemical formulations could directly impact potential exposures to toxic constituents such as metals.
Evidence for ethnicity and location as regulators of the newborn blood metabolome: a monozygous twin study.[Pubmed:38239842]
Front Nutr. 2024 Jan 4;10:1259777.
INTRODUCTION: Monochorionic, diamniotic (MCDA) monozygotic twins share nearly all genetic variation and a common placenta in utero. Despite this, MCDA twins are often discordant for a range of common phenotypes, including early growth and birth weight. As such, MCDA twins represent a unique model to explore variation in early growth attributable primarily to in utero environmental factors. METHODS: MCDA twins with a range of within-pair birth weight discordance were sampled from the peri/postnatal epigenetic twin study (PETS, Melbourne; n = 26 pairs), Beijing twin study (BTS, Beijing; n = 25), and the Chongqing longitudinal twin study (LoTiS, Chongqing; n = 22). All PETS participants were of European-Australian ancestry, while all Chinese participants had Han ancestry. The average of the birth weight difference between the larger and smaller co-twins for all twin pairs was determined and metabolomic profiles of amino acids, TCA cycle intermediates, fatty acids, organic acids, and their derivatives generated from cord blood plasma by gas chromatograph mass spectrometry. Within and between co-twin pair analyses were performed to identify metabolites specifically associated with discordance in birth weight. Multivariable regression and pathway enrichment analyses between different regions were performed to evaluate the geographical effects on the metabolism of MCDA twin pairs. RESULTS: PETS twins showed a markedly different metabolic profile at birth compared to the two Chinese samples. Within-pair analysis revealed an association of glutathione, creatinine, and Levulinic acid with birth weight discordance. Caffeine, phenylalanine, and several saturated fatty acid levels were uniquely elevated in PETS twins and were associated with maternal BMI and average within pair birth weight, in addition to birth weight discordance. LoTiS twins had higher levels of glutathione, tyrosine, and gamma-linolenic acid relative to PETS and BTS twins, potentially associated with eating habits. CONCLUSION: This study highlights the potential role of underlying genetic variation (shared by MZ twins), in utero (non-shared by MZ twins) and location-specific (shared by MZ twins) environmental factors, in regulating the cord blood metabolome of uncomplicated MCDA twins. Future research is needed to unravel these complex relationships that may play a key role in phenotypic metabolic alterations of twins independent of genetic diversity.
Coupling process for preparing biomass-based furfural and levulinic acid from corncob: Extraction, green chemistry and techno-economic assessment.[Pubmed:38211714]
Bioresour Technol. 2024 Feb;394:130301.
The purpose of this study is to design and investigate two coupling processes for acid-catalyzed hydrolysis of corncob, achieving the simultaneous preparation of biomass-based furfural and Levulinic acid (LA). Meanwhile, high concentration and yield of LA were obtained through a situ feeding strategy of pretreated furfural residue with high solids loading (20%, w/v). In Scenario A, 2-methyltetrahydrofuran was selected as the solvent for the LA extraction process compared with the neutralization process in Scenario B. Techno-economic assessment results show that Scenario A is technically feasible and cost-competitive, with an internal rate of return of 21.92%, a net present value of 121 million US dollars, a carbon efficiency of 72%, an environmental factor of 4.38, and a process mass intensity of 32.19. This study will provide new insights for fully utilizing lignocellulosic biomass to prepare renewable energy resources, comprehensively evaluating the economic feasibility, and promoting green and low-carbon development.
Catalytic Conversion of Levulinic Acid into 2-Methyltetrahydrofuran: A Review.[Pubmed:38202825]
Molecules. 2024 Jan 2;29(1):242.
Biomass-derived furanics play a pivotal role in chemical industries, with 2-methyltetrahydrofuran (2-MTHF), a hydrogenated product of Levulinic acid (LA), being particularly significant. 2-MTHF finds valuable applications in the fuel, polymer, and chemical sectors, serving as a key component in P-series biofuel and acknowledged as a renewable solvent for various chemical processes. Numerous research groups have explored catalytic systems to efficiently and selectively convert LA to 2-MTHF, using diverse metal-supported catalysts in different solvents under batch or continuous process conditions. This comprehensive review delves into the impact of metal-supported catalysts, encompassing co-metals and co-catalysts, on the synthesis of 2-MTHF from LA. The article also elucidates the influence of different reaction parameters, such as temperature, type and quantity of hydrogen source, and time. Furthermore, the review provides insights into reaction mechanisms for all documented catalytic systems.
Challenges and Opportunities in the Catalytic Synthesis of Diphenolic Acid and Evaluation of Its Application Potential.[Pubmed:38202709]
Molecules. 2023 Dec 24;29(1):126.
Diphenolic acid, or 4,4-bis(4-hydroxyphenyl)pentanoic acid, represents one of the potentially most interesting bio-products obtainable from the Levulinic acid supply-chain. It represents a valuable candidate for the replacement of bisphenol A, which is strongly questioned for its toxicological issues. Diphenolic acid synthesis involves the condensation reaction between phenol and Levulinic acid and requires the presence of a Bronsted acid as a catalyst. In this review, the state of the art related to the catalytic issues of its synthesis have been critically discussed, with particular attention to the heterogeneous systems, the reference benchmark being represented by the homogeneous acids. The main opportunities in the field of heterogeneous catalysis are deeply discussed, as well as the bottlenecks to be overcome to facilitate diphenolic acid production on an industrial scale. The regioselectivity of the reaction is a critical point because only the p,p'-isomer is of industrial interest; thus, several strategies aiming at the improvement of the selectivity towards this isomer are considered. The future potential of adopting alkyl levulinates, instead of Levulinic acid, as starting materials for the synthesis of new classes of biopolymers, such as new epoxy and phenolic resins and polycarbonates, is also briefly considered.


