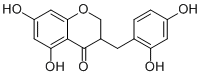
DisporopsinCAS# 1430334-05-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1430334-05-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C16H14O6 | M.Wt | 302.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Disporopsin Dilution Calculator

Disporopsin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate
Catalog No.:BCX0397
CAS No.:148757-94-2
- 17α-Deacetyltanghinin
Catalog No.:BCX0396
CAS No.:111614-46-1
- Levulinic acid
Catalog No.:BCX0395
CAS No.:123-76-2
- 4''-O-Glucosyl-17α-deacetyltanghinin
Catalog No.:BCX0394
CAS No.:114612-75-8
- Tetrahydrorhombifoline
Catalog No.:BCX0393
CAS No.:3382-84-1
- n-Butyl α-D-fructofuranoside
Catalog No.:BCX0392
CAS No.:80971-59-1
- Excoecafolin C
Catalog No.:BCX0391
CAS No.:1643370-00-6
- (2E,6E)-Farnesyl acetate
Catalog No.:BCX0390
CAS No.:4128-17-0
- 4,4'-(1,3-Dimethylbutylidene)diphenol
Catalog No.:BCX0389
CAS No.:6807-17-6
- 14,15,16-Trinorlabda-8(17),11-dien-13-oic acid
Catalog No.:BCX0388
CAS No.:917078-12-7
- Sclerone
Catalog No.:BCX0387
CAS No.:19638-58-5
- Malformin C
Catalog No.:BCX0386
CAS No.:59926-78-2
- 8-Methyldisporopsin
Catalog No.:BCX0399
CAS No.:1671098-51-3
- 8-Methyldisporopsin 4'-methyl ether
Catalog No.:BCX0400
CAS No.:1589545-88-9
- 8-Methyl-2'-deoxydisporopsin
Catalog No.:BCX0401
CAS No.:1671098-52-4
- Disporopsin 4'-methyl ether
Catalog No.:BCX0402
CAS No.:1671098-53-5
- Ichanexic acid
Catalog No.:BCX0403
CAS No.:1044818-57-6
- 3''-Methoxycentrolobol
Catalog No.:BCX0404
CAS No.:811471-19-9
- Kaempferol 7-O-(4''-O-methyl)glucoside
Catalog No.:BCX0405
CAS No.:1092795-48-6
- Suberanilic acid
Catalog No.:BCX0406
CAS No.:149648-52-2
- N1-Phenylsuberamide
Catalog No.:BCX0407
CAS No.:1305124-48-4
- Andrographidine G
Catalog No.:BCX0408
CAS No.:1494685-00-5
- Bisandrographolide G
Catalog No.:BCX0409
CAS No.:2699680-69-6
- Diplacol
Catalog No.:BCX0410
CAS No.:76556-05-3
Revealing the active ingredients and mechanism of P. sibiricumm in non-small-cell lung cancer based on UPLC-Q-TOF-MS/MS, network pharmacology, and molecular docking.[Pubmed:38617965]
Heliyon. 2024 Apr 3;10(7):e29166.
The alcohol extraction of P. sibiricum has exhibited significant inhibitory effects on the production of free radicals and the proliferation of non-small-cell lung carcinoma (NSCLC) A549 cells. Despite the diverse components found in alcohol extraction of P. sibiricum and its multiple targets, the active components and associated targets remain largely unidentified. Hence, there is a need for additional investigation into the pharmacodynamic elements and mechanisms of action. This study aimed to analyze and identify the components responsible for the anti-tumor activity of alcohol extraction from P. sibiricum using UPLC-Q-TOF-MS/MS for the first time. Subsequently, the targets of the active components were predicted using the SwissTargetPrediction database, whereas the targets for NSCLC were sourced from the Online Mendelian Inheritance in Man database (OMIM) and the GeneCards database. Next, the targets of chemical composition were integrated with disease targets via Venny online. GO and KEGG pathway enrichment analyses were performed utilizing DAVID. Subsequently, a network analysis of "components-targets-pathways" was established using Cytoscape 3.8.2 and assessed with the "network analyzer" plug-in. Molecular docking was conducted utilizing Autodock 1.5.6. The study aimed to examine the anti-proliferative impacts and underlying mechanisms of alcohol extraction from P. sibiricum on NSCLC through in vivo and in vitro investigations utilizing an animal model of transplanted tumor, CCK8 assay, cell scratch test, RT-qPCR, and western blotting. The study unveiled that 17 active components extracted from P. sibiricum alcohol demonstrated anti-non-small cell lung cancer (NSCLC) effects through the modulation of 191 targets and various significant signaling pathways. These pathways include Endocrine resistance, PI3K/AKT, Chemical carcinogenesis-receptor activation, Proteoglycans in cancer, EGFR tyrosine kinase inhibitor resistance, AMPK signaling pathway, and other related signaling pathways. Network analysis and molecular docking results indicated that specific compounds such as (25S)-26-O-(beta-d-glucopyranosyl)-furost-5-en3beta,22alpha,26-triol3-O-beta-d-glucopyranosyl-(1-->2)-beta-d-glucopyranosyl-(1-->4)-beta-d-glucopyranoside, Timosaponin H1, Deapi-platycodin D3, (3R)-5,7-dihydroxy-6,8-dimethyl-3-(4'-hydroxybenzyl)-chroman-4-one, Disporopsin, Funkioside F, Kingianoside E, Parisyunnanoside H, and Sibiricoside B primarily targeted 17 key proteins (BCL2, EGFR, ESR1, ESR2, GRB2, IGF1R, JUN, MAP2K1, MAPK14, MAPK8, MDM2, MMP9, mTOR, PIK3CA, RAF1, RPS6KB1, and SRC) collectively. In conclusion, the alcohol extraction of P. sibiricum demonstrated inhibitory effects on cell proliferation, induction of apoptosis, and inhibition of metastasis through various pathways.
Discrimination of Polygonati Rhizoma Species: An Investigation Utilizing High-Performance Liquid Chromatography Fingerprints and Chemometrics.[Pubmed:37291998]
Chem Biodivers. 2023 Jul;20(7):e202300458.
Polygonati Rhizoma has been a famous traditional Chinese medicine (TCM) for two thousand years. It is increasingly being used not just as a traditional herbal medicine but also as a popular functional food. In this study, qualitative and quantitative analysis of PR from three different origins were initially performed using chemical fingerprint and chemometrics methods. Hierarchical cluster analysis (HCA) and Principal component analysis (PCA) were used to classify 60 PR samples from three different origins. The results revealed that the PR samples fell into three clusters related to the origins. In addition, pairwise comparison of varying PR and obtaining chemical markers between different species through the establishment of partial least squares discriminant analysis. Finally, chemical markers 9,13 and 17 were identified by LC/MS as Disporopsin, 5,7-dihydroxy-3-(4'-hydroxybenzyl)-6,8-dimethylchroman-4-one and (3R)-5,7-dihydroxy-3-(4'-hydroxybenzyl)-6-methylchroman-4-one or isomer, respectively. In conclusion, these methods can be applied to identify and distinguish the quality of PR with other original plants and provide novel ideas for evaluating herbal products used in TCM.
Revealing the mechanisms of the bioactive ingredients accumulation in Polygonatum cyrtonema by multiomics analyses.[Pubmed:36466239]
Front Plant Sci. 2022 Nov 16;13:1055721.
Polygonatum cyrtonema is a medicinal and edible herb rich in polysaccharides, steroidal saponins, and flavonoids that has been widely used as a food, vegetable, and medicine over the years. Although previous studies have preliminarily explored the metabolic and transcriptional regulatory mechanisms of the main secondary metabolites in P. cyrtonema, the complex mechanism of microRNA (miRNA)-mediated posttranscriptional regulation remains unclear. Metabolome analysis showed that iso-ophiopogonanone B, (25S)-pratioside D1, Disporopsin, and isodiosgenin-Glc-Glc, which are associated with intermediates in the flavonoids and saponins pathways, were significantly upregulated in the stem and leaf compared with the rhizome, and most saccharides, including arabinose, cellobiose, maltotetraose, and panose, showed the opposite trend, suggesting that they may contribute to the formation and accumulation of the main active ingredients in P. cyrtonema. We found that 4-hydroxymandelonitrile have a relatively good inhibitory effect on alpha-glucosidase, indicating that it may play a role in hypoglycemic functions. Transcriptome and weighted gene coexpression network analysis (WGCNA) were combined to reveal several candidate genes involved in the accumulation of polysaccharides, saponins, and flavonoids, including PcSQLE, PcCYP71A1, PcSUS, PcFK, and PcMYB102. Integrated analyses of miRNAs and messengerRNAs (mRNAs) showed that novel_miR14, novel_miR49, novel_miR75, and aof_miR164 were negatively correlated with alpha-linolenic acid metabolism and the mitogen activated protein kinase (MAPK) signaling pathway, including PcAOS, PcSPLA2, PcFRK1, and PcDELLA, indicating that these miRNAs may coordinately regulate the biosynthesis of other secondary metabolites in P. cyrtonema. These findings will facilitate in-depth research on the functions of these miRNAs and mRNAs related to the main active substances for pathological and biological regulation, which will be beneficial to provide theoretical guidance for the molecular breeding of P. cyrtonema.
Four homoisoflavonoids isolated from traditional Chinese medicine: "gan luo xin".[Pubmed:24993400]
J Asian Nat Prod Res. 2014;16(8):813-8.
Two new homoisoflavonoids, ( +/- )-5,7-dihydroxy-8-methyl-3-(2',4'-dihydroxybenzyl) chroman-4-one (1) and ( +/- )-5,7-dihydroxy-6,8-dimethyl-3-(2',4'-dihydroxybenzyl) chroman-4-one (2), along with two known homoisoflavonoids, 5,7-dihydroxy-6-methyl-3-(2',4'-dihydroxybenzyl)chroman-4-one (3) and Disporopsin (4), were isolated from the EtOAc extract of traditional Chinese medicine--"Gan Luo Xin." Their structures were determined on the basis of spectroscopic analysis (UV, IR, HR-ESI-MS, 1D NMR, and 2D NMR).
[New homoisoflavanones from Polygonatum odoratum (Mill.) Druce].[Pubmed:19806917]
Yao Xue Xue Bao. 2009 Jul;44(7):764-7.
To study chemical constituents of Polygonatum odoratum (Mill.) Druce, the compounds were separated with column chromatography and HPLC. On the basis of physicochemical properties and spectral data, their structures were confirmed. Nine compounds were isolated and identified as 5,7-dihydroxy-6-methoxyl-8-methyl-3-(2',4'-dihydroxybenzyl)chroman-4-one (1), 5,7-dihydroxy-6-methyl-3-(2',4'-dihydroxybenzyl)chroman-4-one (2), 5,7-dihydroxy-6-methoxyl-8-methyl-3-(4'-methoxybenzyl)chroman-4-one (3), Disporopsin (4), chrysoeriol (5), 5,4'-dihydroxy-7-methoxy-6-methylflavone (6), N-trans-feruloyltyramine (7), N-trans-feruloyloctopamine (8), and (+)-syringaresinol (9). Compounds 1-3 are new homoisoflavanones. Compounds 4-9 are isolated from this plant for the first time.
Three new saponins from the fresh rhizomes of Polygonatum kingianum.[Pubmed:19122309]
Chem Pharm Bull (Tokyo). 2009 Jan;57(1):1-4.
Further studies on the fresh rhizomes of Polygonatum kingianum led to the isolation of one new spirostanol saponin (25R)-kingianoside G (1), and two pairs mixture of 25R and 25S stereoisomeric spirostanol saponins (25R, S)-pratioside D1 (2a, 2b) and (25R, S)-kingianoside A (3a, 3b), among them 2b and 3b were new spirostanol saponins, together with another two known compounds, Disporopsin (4) and daucosterol (5). The structures of the new saponins were determined by detailed analysis of their 1D and 2D NMR spectra, and chemical evidences.
Homoisoflavanones from Disporopsis aspera.[Pubmed:16899264]
Phytochemistry. 2006 Oct;67(19):2159-63.
From cytotoxic extracts of the roots of Disporopsis aspera Engl. (Liliaceae) a homoisoflavanone, Disporopsin (3-(2',4'-dihydroxy-benzyl)-5,7-dihydroxy-chroman-4-one) (1) and three rare methyl-homoisoflavanones, 3-(4'-hydroxy-benzyl)-5,7-dihydroxy-6-methyl-chroman-4-one (2), 3-(4'-hydroxy-benzyl)-5,7-dihydroxy-6,8-dimethyl-chroman-4-one (3) and 3-(4'-hydroxy-benzyl)-5,7-dihydroxy-6-methyl-8-methoxy-chroman-4- one (4) along with five other known compounds, N-trans-feruloyl tyramine (5), adenine (6), 5-(hydroxymethyl)-2-furfural (7), beta-sitosterol (8) and beta-sitosteryl glucopyranoside (9) were isolated. The structures of compounds 1-2 were elucidated by spectral data (1, 2-D NMR and EIMS). The four homoisoflavanones (1-4) were found to be cytotoxic against a series of human cancer cell lines (HCT15, T24S, MCF7, Bowes, A549 and K562) with IC(50) ranging from 15 to 200 microM. Possible biosynthesis routes for homoisoflavonoids (1-4) are discussed.


