DiplacolCAS# 76556-05-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 76556-05-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
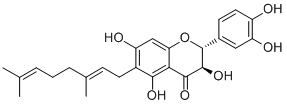
| Formula | C25H28O7 | M.Wt | 440.53 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Diplacol Dilution Calculator

Diplacol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.27 mL | 11.35 mL | 22.6999 mL | 45.3999 mL | 56.7498 mL |
| 5 mM | 0.454 mL | 2.27 mL | 4.54 mL | 9.08 mL | 11.35 mL |
| 10 mM | 0.227 mL | 1.135 mL | 2.27 mL | 4.54 mL | 5.675 mL |
| 50 mM | 0.0454 mL | 0.227 mL | 0.454 mL | 0.908 mL | 1.135 mL |
| 100 mM | 0.0227 mL | 0.1135 mL | 0.227 mL | 0.454 mL | 0.5675 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bisandrographolide G
Catalog No.:BCX0409
CAS No.:2699680-69-6
- Andrographidine G
Catalog No.:BCX0408
CAS No.:1494685-00-5
- N1-Phenylsuberamide
Catalog No.:BCX0407
CAS No.:1305124-48-4
- Suberanilic acid
Catalog No.:BCX0406
CAS No.:149648-52-2
- Kaempferol 7-O-(4''-O-methyl)glucoside
Catalog No.:BCX0405
CAS No.:1092795-48-6
- 3''-Methoxycentrolobol
Catalog No.:BCX0404
CAS No.:811471-19-9
- Ichanexic acid
Catalog No.:BCX0403
CAS No.:1044818-57-6
- Disporopsin 4'-methyl ether
Catalog No.:BCX0402
CAS No.:1671098-53-5
- 8-Methyl-2'-deoxydisporopsin
Catalog No.:BCX0401
CAS No.:1671098-52-4
- 8-Methyldisporopsin 4'-methyl ether
Catalog No.:BCX0400
CAS No.:1589545-88-9
- 8-Methyldisporopsin
Catalog No.:BCX0399
CAS No.:1671098-51-3
- Disporopsin
Catalog No.:BCX0398
CAS No.:1430334-05-6
- 4''-O-Glucosyl-17β-deacetyltanghinin
Catalog No.:BCX0411
CAS No.:114613-60-4
- 6''-O-(3-Hydroxy-3-methylglutaroyl)astragalin
Catalog No.:BCX0412
CAS No.:157407-84-6
- 12α-Methoxyabietic acid
Catalog No.:BCX0413
CAS No.:83905-80-0
- 4-(5-Oxotetrahydrofuran-2-yl)-N-phenylbutanamide
Catalog No.:BCX0414
CAS No.:100718-44-3
- (S)-5-Hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)heptan-3-one
Catalog No.:BCX0415
CAS No.:619297-56-2
- 5,6,7,2'-Tetramethoxyisoflavone
Catalog No.:BCX0416
CAS No.:100211-04-9
- Nomilin 17-O-glucoside
Catalog No.:BCX0417
CAS No.:123564-62-5
- Lycoperodine 1
Catalog No.:BCX0418
CAS No.:6052-68-2
- 5α,6α-Epoxyergosta-8(14),22-diene-3β,7α-diol
Catalog No.:BCX0419
CAS No.:22259-18-3
- 20(R)-Hydroxypregn-4-en-3-one
Catalog No.:BCX0420
CAS No.:145-15-3
- 5α,6α-Epoxyergosta-8,22-diene-3β,7α-diol
Catalog No.:BCX0421
CAS No.:16250-61-6
- Azoxystrobin
Catalog No.:BCX0422
CAS No.:131860-33-8
Perspectives on antimicrobial properties of Paulownia tomentosa Steud. fruit products in the control of Staphylococcus aureus infections.[Pubmed:37979817]
J Ethnopharmacol. 2024 Mar 1;321:117461.
ETHNOPHARMACOLOGICAL RELEVANCE: Paulownia tomentosa Steud. (P. tomentosa) is a medium-sized tree traditionally used in Chinese folk medicine for the treatment of infectious diseases. It is a rich source of prenylated phenolic compounds that have been extensively studied for their promising biological activities. AIM OF THE STUDY: Due to the increasing development of antibiotic resistance, our study investigated plant-derived natural products from the fruits of P. tomentosa that could control Staphylococcus aureus infections with novel targets/modes of action and reduce antimicrobial resistance. MATERIALS AND METHODS: The ethanolic extract was fractionated and detected by liquid chromatography. The antistaphylococcal effects of the plant formulations were studied in detail in vitro by various biological methods, including microdilution methods for minimum inhibitory concentration (MIC), the checkerboard titration technique for synergy assay, fluorescence measurements for membrane disruption experiments, autoinducer-2-mediated bioassay for quorum sensing inhibition, and counting of colony-forming units for relative adhesion. Morphology was examined by transmission electron microscopy. RESULTS: Total ethanolic extract and chloroform fraction showed MICs of 128 and 32 mug/mL, respectively. Diplacol, diplacone, and 3'-O-methyl-5'-hydroxydiplacone inhibited S. aureus growth in the range of 8-16 mug/mL. Synergistic potential was shown in combination with mupirocin and fusidic acid. The ethanolic extract and the chloroform fraction destroyed the cell membranes by 91.61% and 79.46%, respectively, while the pure compounds were less active. The ethanolic extract and the pure compounds reduced the number of adhered cells to 47.33-10.26% compared to the untreated control. All tested plant formulations, except diplacone, inhibited quorum sensing of S. aureus. Transmission electron microscopy showed deformation of S. aureus cells. CONCLUSIONS: The products from the fruit of P. tomentosa showed antimicrobial properties against S. aureus alone and in combination with antibiotics. By affecting intracellular targets, geranylated flavonoids proposed novel approaches in the control of staphylococcal infections.
Discovery of putative inhibitors against main drivers of SARS-CoV-2 infection: Insight from quantum mechanical evaluation and molecular modeling.[Pubmed:36304744]
Front Chem. 2022 Oct 11;10:964446.
SARS-CoV-2 triggered a worldwide medical crisis, affecting the world's social, emotional, physical, and economic equilibrium. However, treatment choices and targets for finding a solution to COVID-19's threat are becoming limited. A viable approach to combating the threat of COVID-19 is by unraveling newer pharmacological and therapeutic targets pertinent in the viral survival and adaptive mechanisms within the host biological milieu which in turn provides the opportunity to discover promising inhibitors against COVID-19. Therefore, using high-throughput virtual screening, manually curated compounds library from some medicinal plants were screened against four main drivers of SARS-CoV-2 (spike glycoprotein, PLpro, 3CLpro, and RdRp). In addition, molecular docking, Prime MM/GBSA (molecular mechanics/generalized Born surface area) analysis, molecular dynamics (MD) simulation, and drug-likeness screening were performed to identify potential phytodrugs candidates for COVID-19 treatment. In support of these approaches, we used a series of computational modeling approaches to develop therapeutic agents against COVID-19. Out of the screened compounds against the selected SARS-CoV-2 therapeutic targets, only compounds with no violations of Lipinski's rule of five and high binding affinity were considered as potential anti-COVID-19 drugs. However, lonchocarpol A, Diplacol, and broussonol E (lead compounds) were recorded as the best compounds that satisfied this requirement, and they demonstrated their highest binding affinity against 3CLpro. Therefore, the 3CLpro target and the three lead compounds were selected for further analysis. Through protein-ligand mapping and interaction profiling, the three lead compounds formed essential interactions such as hydrogen bonds and hydrophobic interactions with amino acid residues at the binding pocket of 3CLpro. The key amino acid residues at the 3CLpro active site participating in the hydrophobic and polar inter/intra molecular interaction were TYR54, PRO52, CYS44, MET49, MET165, CYS145, HIS41, THR26, THR25, GLN189, and THR190. The compounds demonstrated stable protein-ligand complexes in the active site of the target (3CLpro) over a 100 ns simulation period with stable protein-ligand trajectories. Drug-likeness screening shows that the compounds are druggable molecules, and the toxicity descriptors established that the compounds demonstrated a good biosafety profile. Furthermore, the compounds were chemically reactive with promising molecular electron potential properties. Collectively, we propose that the discovered lead compounds may open the way for establishing phytodrugs to manage COVID-19 pandemics and new chemical libraries to prevent COVID-19 entry into the host based on the findings of this computational investigation.
Geranylated flavanones from the secretion on the surface of the immature fruits of Paulownia tomentosa.[Pubmed:18206191]
Phytochemistry. 2008 Mar;69(5):1234-41.
Chemical investigation of the methanol extract of the viscous secretion on the surface of immature fruits of Paulownia tomentosa furnished nine geranylated flavanones, 6-geranyl-5,7-dihydroxy-3',4'-dimethoxyflavanone (1), 6-geranyl-3',5,7-trihydroxy-4'-methoxyflavanone (2), 6-geranyl-4',5,7-trihydroxy-3',5'-dimethoxyflavanone (3), 6-geranyl-4',5,5',7-tetrahydroxy-3'-methoxyflavanone (4), 6-geranyl-3,3',5,7-tetrahydroxy-4'-methoxyflavanone (5), 4',5,5',7-tetrahydroxy-6-[6-hydroxy-3,7-dimethyl-2(E),7-octadienyl]-3'-methoxyflavanone (6), 3,3',4',5,7-pentahydroxy-6-[6-hydroxy-3,7-dimethyl-2(E),7-octadienyl]flavanone (7), 3,3',4',5,7-pentahydroxy-6-[7-hydroxy-3,7-dimethyl-2(E)-octenyl]flavanone (8), and 3,4',5,5',7-pentahydroxy-3'-methoxy-6-(3-methyl-2-butenyl)flavanone (9), along with six known geranylated flavanones. Among these, compounds 4, 6-9 and the known 6-geranyl-3',4',5,7-tetraahydroxyflavanone (diplacone), 6-geranyl-3,3',4',5,7-pentahydroxyflavanone (Diplacol) and 3',4',5,7-pentahydroxy-6-[7-hydroxy-3,7-dimethyl-2(E)-octenyl]flavanone showed potent radical scavenging effects towards DPPH radicals.
Antiproliferative prenylated stilbenes and flavonoids from Macaranga alnifolia from the Madagascar rainforest.[Pubmed:17326683]
J Nat Prod. 2007 Mar;70(3):342-6.
Bioassay-guided fractionation of an extract of the fruit of Macaranga alnifolia from Madagascar led to the isolation of four new prenylated stilbenes, schweinfurthins E-H (1-4), and one new geranylated dihydroflavonol, alnifoliol (5). The known prenylated stilbene vedelianin (6) and the known geranylated flavonoids bonanniol A (7), Diplacol (8), bonannione A (9), and diplacone (10) were also isolated. All 10 compounds were tested for antiproliferative activity in the A2780 human ovarian cancer cell line assay. Vedelianin (IC50 = 0.13 microM) exhibited the greatest activity among all isolates, while schweinfurthin E (IC50 = 0.26 microM) was the most potent of the new compounds.
Cytotoxic compounds of Schizolaena hystrix from the Madagascar rainforest.[Pubmed:16902871]
Planta Med. 2006 Oct;72(13):1235-8.
Bioassay-guided fractionation of a crude ethanol extract from a Madagascar collection of Schizolaena hystrix afforded the two new long-chain compounds, 3 S-acetoxyeicosanoic acid ethyl ester ( 1) and 3 S-acetoxydoeicosanoic acid ( 2), and the known long-chain compound 3 S-acetoxyeicosanoic acid ( 3). In addition, the long-chain alcohol 1-hydroxydodecan-2-one ( 7), as well as the new flavonoid schizolaenone C ( 4) and the two known flavonoids Diplacol ( 5) and 3'-prenylnaringenin ( 6) were isolated from a methanol extract of the same plant. Isolation and structure elucidation of the novel compounds and the cytotoxicities of all the isolates are reported.
C-geranyl compounds from Mimulus clevelandii.[Pubmed:8778239]
J Nat Prod. 1996 May;59(5):495-7.
Fractionation of the MeCOEt extract of Mimulus clevelandii yielded the novel 4-geranyl-5-hydroxy-2(3H)-benzofuranone (1) and the five known 6-geranylflavanones diplacone (2a), 3'-O-methyldiplacone (2b), Diplacol (2c), mimulone (2d), and 3'-O-methylDiplacol (2e). 2D-NMR methods required revision of assignments for diplacone and Diplacol and resolved the uncertainty in the site of methylation for the methyl ethers.


