Melanocyte stimulating hormone release inhibiting factorMSH release-inhibiting factor CAS# 2002-44-0 |

- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2002-44-0 | SDF | Download SDF |
| PubChem ID | 92910 | Appearance | Powder |
| Formula | C13H24N4O3 | M.Wt | 284.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pro-Leu-Gly | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | PLG (Modifications: Gly-3 = C-terminal amide) | ||
| Chemical Name | (2S)-N-[(2S)-1-[(2-amino-2-oxoethyl)amino]-4-methyl-1-oxopentan-2-yl]pyrrolidine-2-carboxamide | ||
| SMILES | CC(C)CC(C(=O)NCC(=O)N)NC(=O)C1CCCN1 | ||
| Standard InChIKey | NOOJLZTTWSNHOX-UWVGGRQHSA-N | ||
| Standard InChI | InChI=1S/C13H24N4O3/c1-8(2)6-10(12(19)16-7-11(14)18)17-13(20)9-4-3-5-15-9/h8-10,15H,3-7H2,1-2H3,(H2,14,18)(H,16,19)(H,17,20)/t9-,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of melanocyte-stimulating hormone (MSH) release. Blocks the release of α-MSH, increases brain dopamine levels and antagonizes physiological and behavioral opioid effects in vivo. |

Melanocyte stimulating hormone release inhibiting factor Dilution Calculator

Melanocyte stimulating hormone release inhibiting factor Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Melanocyte stimulating hormone release inhibiting factor,(C13H24N4O3), a tri-peptide with the sequence H2N-Pro-Leu-Gly-amide, MW= 284.35. Melanocyte-inhibiting factor (also known as Pro-Leu-Gly-NH2, Melanostatin, MSH release-inhibiting hormone or MIF-1) is an endogenous peptide fragment derived from cleavage of the hormone oxytocin, but having generally different actions in the body(1). MIF-1 produces multiple effects, both blocking the effects of opioid receptor activation(2), while at the same time acting as a positive allosteric modulator of the D2 and D4 dopamine receptor subtypes, as well as inhibiting release of other neuropeptides such as alpha-MSH, and potentiating melatonin activity(3). MIF-1 is unusually resistant to metabolism in the bloodstream, and crosses the blood–brain barrier easily, though it is poorly active orally and is usually injected.
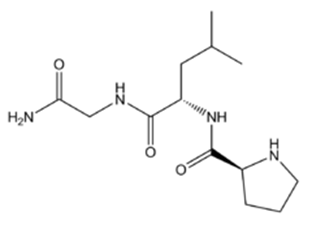
Figure1 Formula of Melanocyte stimulating hormone release inhibiting factor
Ref:
1. Celis ME, Taleisnik S, Walter R (July 1971). "Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone". Proceedings of the National Academy of Sciences of the United States of America 68 (7): 1428–33.
2. Contreras PC, Takemori AE (June 1984). "Effect of prolyl-leucyl-glycinamide and alpha-melanocyte-stimulating hormone on levorphanol-induced analgesia, tolerance and dependence". Life Sciences 34 (26): 2559–66.
3. Sandyk R (May 1990). "MIF-induced augmentation of melatonin functions: possible relevance to mechanisms of action of MIF-1 inmovement disorders". The International Journal of Neuroscience 52 (1-2): 59–65.
- Z-Arg(Pbf)-OH.CHA
Catalog No.:BCC3064
CAS No.:200190-89-2
- CGP 3466B maleate
Catalog No.:BCC5955
CAS No.:200189-97-5
- Fmoc-Arg(Pbf)-OPfp
Catalog No.:BCC3041
CAS No.:200132-16-7
- Dehydrochromolaenin
Catalog No.:BCN4072
CAS No.:20013-76-7
- Pyrocurzerenone
Catalog No.:BCN4067
CAS No.:20013-75-6
- Isoscoparin
Catalog No.:BCN7845
CAS No.:20013-23-4
- Caulophylline hydriodide
Catalog No.:BCC8141
CAS No.:20013-22-3
- 3'-Methoxydaidzin
Catalog No.:BCN7720
CAS No.:200127-80-6
- Boc-Arg(Pbf)-OH
Catalog No.:BCC3066
CAS No.:200124-22-7
- SKF 38393 hydrobromide
Catalog No.:BCC6848
CAS No.:20012-10-6
- H-D-Arg(Pbf)-OH
Catalog No.:BCC2872
CAS No.:200116-81-0
- H-Arg(Pbf)-OH
Catalog No.:BCC2866
CAS No.:200115-86-2
- Boc-D-Asp(OtBu)-OH.DCHA
Catalog No.:BCC3373
CAS No.:200334-95-8
- Fmoc-D-Asp(OtBu)-Opfp
Catalog No.:BCC3472
CAS No.:200335-75-7
- Fmoc-D-Cit-OH
Catalog No.:BCC3181
CAS No.:200344-33-8
- Fmoc-D-Cys(Trt)-OPfp
Catalog No.:BCC3482
CAS No.:200395-72-8
- 6-Chloroguanine riboside
Catalog No.:BCC8771
CAS No.:2004-07-1
- UB 165 fumarate
Catalog No.:BCC5746
CAS No.:200432-86-6
- Picralinal
Catalog No.:BCN4878
CAS No.:20045-06-1
- GMX1778 (CHS828)
Catalog No.:BCC6527
CAS No.:200484-11-3
- Hotrienol
Catalog No.:BCN6340
CAS No.:20053-88-7
- Fmoc-D-Glu(OtBu)-OPfp
Catalog No.:BCC3497
CAS No.:200616-21-3
- Fmoc-N-Me-Glu(OtBu)-OH
Catalog No.:BCC3213
CAS No.:200616-40-6
- Fmoc-D-Gln-OPfp
Catalog No.:BCC3487
CAS No.:200622-33-9
Melanocyte-stimulating hormone release-inhibiting factor-1 (MIF-1) can be formed from Tyr-MIF-1 in brain mitochondria but not in brain homogenate.[Pubmed:7891114]
J Neurochem. 1995 Apr;64(4):1855-9.
Two samples of the peptide tyrosine-melanocyte-stimulating hormone release-inhibiting factor-1 (Tyr-MIF-1; Tyr-Pro-Leu-Gly-NH2) were tritiated on different amino acids (Tyr or Pro) and incubated together at 37 degrees C with fractions of rat brain. The amount of intact tetrapeptide remaining was determined by HPLC. By 3 min, most of the Tyr-MIF-1 was degraded. Because similar amounts of [3H]Pro and [3H]Tyr appeared after incubation of the Tyr-MIF-1 peptides in brain homogenate, even as early as 30 s, examination of only this crude preparation would misleadingly indicate that Tyr-MIF-1 is not a precursor of melanocyte-stimulating hormone release-inhibiting factor-1 (MIF-1; Pro-Leu-Gly-NH2) in brain tissue. However, incubation of the mitochondrial fractions of brain under the same conditions resulted in more than three times as much [3H]Tyr being formed as [3H]Pro, with accompanying accumulation of MIF-1. Addition of excess MIF-1 to the mitochondrial fraction completely suppressed the formation of MIF-1 and more than doubled the amount of Tyr-MIF-1 remaining intact. When Tyr-MIF-1 tritiated only on the Tyr was added to the mitochondrial fraction, the main peaks of radioactivity appeared only at the positions of Tyr and Tyr-MIF-1, not at the position of Tyr-Pro. The results indicate that Tyr-MIF-1 can serve as a precursor of MIF-1 in brain mitochondria, an effect not evident when crude brain homogenate is used.
The purification of a bovine kidney enzyme which cleaves melanocyte-stimulating hormone-release inhibiting factor.[Pubmed:31186]
Biochim Biophys Acta. 1978 Nov 10;527(1):282-8.
An enzyme which catalyzes the hydrolysis of L-prolyl-L-leucylglycinamide, the factor which inhibits the release of melanocyte-stimulating hormone, was purified 189-fold from bovine kidney in a 5% yield. The molecular weight of the enzyme on gel filtration was estimated to be 300 000 and its isoelectric point was found to be pH 4.1. The single component seen on sodium dodecyl sulphate-gel electrophoresis was estimated to have a molecular weight of 56 000, indicating that the native enzyme may be a pentamer or hexamer. The enzyme could clearly be distinguished from other prolyl-cleaving enzymes.
Behavioral effects of melanocyte stimulating hormone release-inhibiting factor-1 (MIF-1).[Pubmed:6136015]
Neurosci Biobehav Rev. 1983 Summer;7(2):257-62.
Consideration of the isolation, structure, localization, and behavioral effects of Melanocyte stimulating hormone release inhibiting factor (MIF-1) is followed by a review of its opiate antagonistic and clinical effects. Evidence pertaining to various hypotheses offered in explanation of these behavioral effects is examined and evaluated. It is concluded that MIF-1 affects behavior in many instances with possible antagonistic effects as well as clinical possibilities.
Isolation of tyrosine-melanocyte-stimulating hormone release-inhibiting factor 1 from bovine brain tissue.[Pubmed:2563371]
J Biol Chem. 1989 Feb 5;264(4):2175-9.
Although Tyr-melanocyte-stimulating hormone release-inhibiting factor 1 (MIF-1) (Tyr-Pro-Leu-Gly-NH2) can exert a number of biological actions in the brain and elsewhere, it has never been isolated from any tissue. Accordingly, we attempted to purify it from acetic acid extracts of bovine brain tissue by gel filtration chromatography and several different high performance liquid chromatographic systems. Peptide content was followed by a specific and sensitive radioimmunoassay with an antibody that was generated against synthetic Tyr-MIF-1. In each of the five applied high performance liquid chromatographic systems, the immunoreactive fractions coincided exactly with the elution time of synthetic Tyr-MIF-1 in the control runnings. The structure of the isolated peptide was identified by microsequence analysis as the tetrapeptide Tyr-Pro-Leu-Gly-NH2 and shown to be biologically active.
Inhibitory influences of FMRFamide and PLG on stress-induced opioid analgesia and activity.[Pubmed:2871903]
Brain Res. 1986 May 7;372(2):370-4.
The effects of i.c.v. administration of the peptide FMRFamide (Phe-Met-Arg-Phe-NH2), as well as i.p. injections of PLG (Pro-Leu-Gly-NH2) and the opiate antagonist, naloxone, on immobilization-induced analgesia and locomotor activity were examined in CF-1 and C57BL strains of mice. Both naloxone (1.0 mg/kg) and FMRFamide (0.10-1.0 microgram) blocked the experimentally induced analgesia and activity, whereas PLG (0.10-10 mg/kg) suppressed only analgesia. These results indicate that FMRFamide (or FMRFamide-like neuropeptides) and PLG may function as differential antagonists of the behavioral and physiological consequences of endogenous opioid activation.


