Moracin OCAS# 123702-97-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 123702-97-6 | SDF | Download SDF |
| PubChem ID | 42604718 | Appearance | Powder |
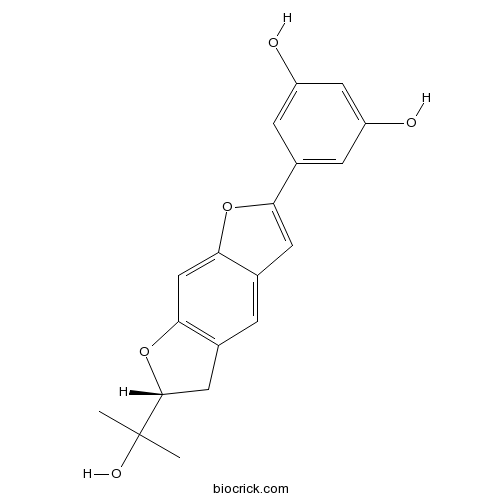
| Formula | C19H18O5 | M.Wt | 326.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[(6R)-6-(2-hydroxypropan-2-yl)-5,6-dihydrofuro[3,2-f][1]benzofuran-2-yl]benzene-1,3-diol | ||
| SMILES | CC(C)(C1CC2=C(O1)C=C3C(=C2)C=C(O3)C4=CC(=CC(=C4)O)O)O | ||
| Standard InChIKey | HMTMYIWMPJSCAZ-GOSISDBHSA-N | ||
| Standard InChI | InChI=1S/C19H18O5/c1-19(2,22)18-7-11-3-10-6-15(23-16(10)9-17(11)24-18)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3/t18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Moracin O and moracin P exhibit potent in vitro inhibitory activity against hypoxia-inducible factor (HIF-1), which is a key mediator during adaptation of cancer cells to tumour hypoxia. 2. Moracin O shows significant neuroprotective activity against glutamate-induced cell death in SK-N-SH cells. 3. Moracin O demonstrates a remarkable inhibition of the acetic acid-induced pain. 4. Moracin O has a strong protective influence against doxorubicin-induced cardiomyopathy in H9c2 cells with the EC50 value of 4.5 ± 1.3 uM. |
| Targets | HIF |

Moracin O Dilution Calculator

Moracin O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0637 mL | 15.3186 mL | 30.6373 mL | 61.2745 mL | 76.5931 mL |
| 5 mM | 0.6127 mL | 3.0637 mL | 6.1275 mL | 12.2549 mL | 15.3186 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0637 mL | 6.1275 mL | 7.6593 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6127 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6127 mL | 0.7659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kuwanol C
Catalog No.:BCN3941
CAS No.:123702-94-3
- CGP 36216 hydrochloride
Catalog No.:BCC7605
CAS No.:123691-29-2
- CGP 46381
Catalog No.:BCC6990
CAS No.:123691-14-5
- CGP 35348
Catalog No.:BCC6988
CAS No.:123690-79-9
- Bongardol
Catalog No.:BCN6124
CAS No.:123690-76-6
- Trigoxyphin A
Catalog No.:BCN6875
CAS No.:1236874-00-2
- Pimasertib (AS-703026)
Catalog No.:BCC2529
CAS No.:1236699-92-5
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- NS 398
Catalog No.:BCC6857
CAS No.:123653-11-2
- Fmoc-Glu(OBzl)-OH
Catalog No.:BCC3493
CAS No.:123639-61-2
- Tenofovir maleate
Catalog No.:BCC4262
CAS No.:1236287-04-9
- Diosbulbin L
Catalog No.:BCN7305
CAS No.:1236285-87-2
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- Aucherine
Catalog No.:BCN2058
CAS No.:123715-12-8
- Escin IA
Catalog No.:BCN3862
CAS No.:123748-68-5
- ML 786 dihydrochloride
Catalog No.:BCC7997
CAS No.:1237536-18-3
- QNZ 46
Catalog No.:BCC6292
CAS No.:1237744-13-6
- Hydroxyevodiamine
Catalog No.:BCN2491
CAS No.:1238-43-3
- Hopeachinol B
Catalog No.:BCN3445
CAS No.:1238083-45-8
- Kazinol U
Catalog No.:BCN4720
CAS No.:1238116-48-7
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
Bioactive Benzofuran Derivatives from Cortex Mori Radicis, and Their Neuroprotective and Analgesic Activities Mediated by mGluR(1).[Pubmed:28208727]
Molecules. 2017 Feb 8;22(2). pii: molecules22020236.
Four new benzofuran-type stilbene glycosides and 14 known compounds including 8 benzofuran-type stilbenes and 6 flavonoids were isolated from the traditional Chinese medicine, Cortex Mori Radicis. The new compounds were identified as (9R)-moracin P 3'-O-alpha-l-arabinopyranoside (1), (9R)-moracin P 9-O-beta-d-glucopyranoside (2), (9R)-moracin P 3'-O-beta-d-glucopyranoside (3), and (9R)-Moracin O 10-O-beta-d-glucopyranoside (4) based on the spectroscopic interpretation and chemical analysis. Three benzofuran-type stilbenes, Moracin O (5), R (7), and P (8) showed significant neuroprotective activity against glutamate-induced cell death in SK-N-SH cells. In addition, Moracin O (5) and P (8) also demonstrated a remarkable inhibition of the acetic acid-induced pain. The molecular docking with metabotropic glutamate receptor 1 (mGluR(1)) results indicated that these neuroprotective benzofuran-type stilbenes might be the active analgesic components of the genus Morus, and acted by mediating the mGluR(1) pathway.
HIF-1alpha inhibitors: synthesis and biological evaluation of novel moracin O and P analogues.[Pubmed:21481991]
Eur J Med Chem. 2011 Jun;46(6):2386-96.
The natural products moracins O and P exhibited potent in vitro inhibitory activity against hypoxia-inducible factor (HIF-1), which is a key mediator during adaptation of cancer cells to tumour hypoxia. Systematic variations of the structures of benzofuran type moracins were made and structure-activity relationship analysis showed the importance of the 2-arylbenzofuran ring and the (R)-configuration of the core scaffold. Further evaluation of the representative compound 5 showed its inhibitory effect on HIF-1alpha protein accumulation and target gene expression under hypoxia.
Phenolic constituents from the root bark of Morus alba L. and their cardioprotective activity in vitro.[Pubmed:27974159]
Phytochemistry. 2017 Mar;135:128-134.
A flavanone C-glycoside, steppogenin-5'-C-beta-D-glucopyranoside, six prenylated 2-arylbenzofuran derivatives, Moracin O-3''-O-beta-D-glucopyranoside, Moracin O-3'-O-beta-D-xylopyranoside, moracin P-2''-O-beta-D-glucopyranoside, moracin P-3'-O-beta-D-glucopyranoside, moracin P-3'-O-alpha-L-arabinopyranoside and moracin P-3'-O-[beta-D-glucopyranosyl-(1 --> 2)]-alpha-L-arabinopyranoside, two phenolic acids, 2,4-dihydroxy-5-(4-hydroxybenzyl) benzoic acid and 2,4-dihydroxy-5-(3,4-dihydroxybenzyl) benzoic acid, as well as three known compounds, moracinoside C, Moracin O, and moracin P were isolated from the root bark of Morus alba L. Their structures were ascertained on the basis of spectroscopic evidence. The protective effects of the compounds against doxorubicin-induced cardiomyopathy in H9c2 cells was investigated in vitro. Of all of the isolated compounds, moracin P-3'-O-beta-D-glucopyranoside, Moracin O and moracin P had a strong protective influence against doxorubicin-induced cell death with EC50 values of 9.5 +/- 2.6, 4.5 +/- 1.3, and 8.8 +/- 2.4 muM, respectively.
The first total synthesis of moracin O and moracin P, and establishment of the absolute configuration of moracin O.[Pubmed:19319432]
Chem Commun (Camb). 2009 Apr 14;(14):1879-81.
The first total synthesis of the naturally occurring benzofurans, moracins O and P was achieved using a Sonogashira cross coupling reaction followed by in situ cyclization, and the absolute configuration of natural Moracin O was established.


