UNC0321G9a inhibitor CAS# 1238673-32-9 |
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- UNC0638
Catalog No.:BCC1135
CAS No.:1255580-76-7
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
- UNC0379
Catalog No.:BCC8055
CAS No.:1620401-82-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1238673-32-9 | SDF | Download SDF |
| PubChem ID | 46901937 | Appearance | Powder |
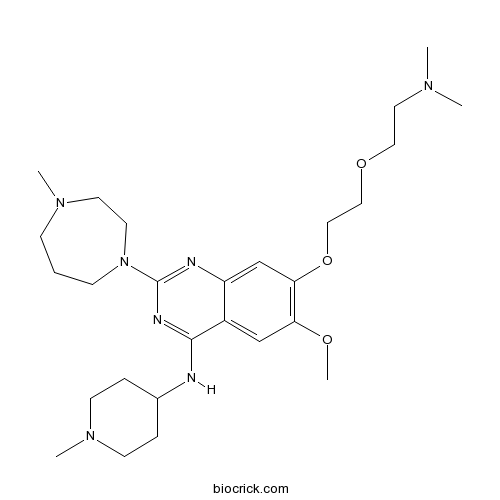
| Formula | C27H45N7O3 | M.Wt | 515.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (60.11 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 7-[2-[2-(dimethylamino)ethoxy]ethoxy]-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine | ||
| SMILES | CN1CCCN(CC1)C2=NC3=CC(=C(C=C3C(=N2)NC4CCN(CC4)C)OC)OCCOCCN(C)C | ||
| Standard InChIKey | AULLUGALUBVBDD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H45N7O3/c1-31(2)15-16-36-17-18-37-25-20-23-22(19-24(25)35-5)26(28-21-7-11-33(4)12-8-21)30-27(29-23)34-10-6-9-32(3)13-14-34/h19-21H,6-18H2,1-5H3,(H,28,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

UNC0321 Dilution Calculator

UNC0321 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9391 mL | 9.6957 mL | 19.3915 mL | 38.783 mL | 48.4787 mL |
| 5 mM | 0.3878 mL | 1.9391 mL | 3.8783 mL | 7.7566 mL | 9.6957 mL |
| 10 mM | 0.1939 mL | 0.9696 mL | 1.9391 mL | 3.8783 mL | 4.8479 mL |
| 50 mM | 0.0388 mL | 0.1939 mL | 0.3878 mL | 0.7757 mL | 0.9696 mL |
| 100 mM | 0.0194 mL | 0.097 mL | 0.1939 mL | 0.3878 mL | 0.4848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
UNC0321 is the most potent small-molecule inhibitor of protein lysine methyltransferase G9a, an enzyme methylating lysine 9 of histone H3 (H3K9) and lysine 373 (K373) of p53, with the inhibition constant Ki value of 63 nM and the half maximal inhibition concentration IC50 values of 9 nM in enzyme-coupled SAH detection assay (ECSD) and 6 nM in chemiluminescence-based oxygen tunneling assay (CLOT) respectively [1].
UNC0321 has also been found to inhibit G9a like protein (GLP), an H3K9 and p53 K373 methyltransferase sharing 80% sequence identity in SET domain with G9a, with IC50 values of 15 nM (ECSD) and 23 nM (CLOT) respectively [1].
Reference
References:
[1] Liu F, Chen X, Allali-Hassani A, Quinn AM, Wigle TJ, Wasney GA, Dong A, Senisterra G, Chau I, Siarheyeva A, Norris JL, Kireev DB, Jadhav A, Herold JM, Janzen WP, Arrowsmith CH, Frye SV, Brown PJ, Simeonov A, Vedadi M, Jin J. Protein lysine methyltransferase G9a inhibitors: design, synthesis, and structure activity relationships of 2,4-diamino-7-aminoalkoxy-quinazolines. J Med Chem. 2010 Aug 12;53(15):5844-57. doi: 10.1021/jm100478y.
- Kazinol U
Catalog No.:BCN4720
CAS No.:1238116-48-7
- Hopeachinol B
Catalog No.:BCN3445
CAS No.:1238083-45-8
- Hydroxyevodiamine
Catalog No.:BCN2491
CAS No.:1238-43-3
- QNZ 46
Catalog No.:BCC6292
CAS No.:1237744-13-6
- ML 786 dihydrochloride
Catalog No.:BCC7997
CAS No.:1237536-18-3
- Escin IA
Catalog No.:BCN3862
CAS No.:123748-68-5
- Aucherine
Catalog No.:BCN2058
CAS No.:123715-12-8
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- Moracin O
Catalog No.:BCN4004
CAS No.:123702-97-6
- Kuwanol C
Catalog No.:BCN3941
CAS No.:123702-94-3
- CGP 36216 hydrochloride
Catalog No.:BCC7605
CAS No.:123691-29-2
- CGP 46381
Catalog No.:BCC6990
CAS No.:123691-14-5
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
Optimization of cellular activity of G9a inhibitors 7-aminoalkoxy-quinazolines.[Pubmed:21780790]
J Med Chem. 2011 Sep 8;54(17):6139-50.
Protein lysine methyltransferase G9a plays key roles in the transcriptional repression of a variety of genes via dimethylation of lysine 9 on histone H3 (H3K9me2) of chromatin as well as dimethylation of nonhistone proteins including tumor suppressor p53. We previously reported the discovery of UNC0321 (3), the most potent G9a inhibitor to date, via structure-based design and structure-activity relationship (SAR) exploration of the quinazoline scaffold represented by BIX01294 (1). Despite its very high in vitro potency, compound 3 lacks sufficient cellular potency. The design and synthesis of several generations of new analogues aimed at improving cell membrane permeability while maintaining high in vitro potency resulted in the discovery of a number of novel G9a inhibitors such as UNC0646 (6) and UNC0631 (7) with excellent potency in a variety of cell lines and excellent separation of functional potency versus cell toxicity. The design, synthesis, and cellular SAR of these potent G9a inhibitors are described.
A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite Plasmodium falciparum.[Pubmed:25993442]
PLoS Genet. 2015 May 19;11(5):e1005234.
Mutually exclusive gene expression, whereby only one member of a multi-gene family is selected for activation, is used by the malaria parasite Plasmodium falciparum to escape the human immune system and perpetuate long-term, chronic infections. A family of genes called var encodes the chief antigenic and virulence determinant of P. falciparum malaria. var genes are transcribed in a mutually exclusive manner, with switching between active genes resulting in antigenic variation. While recent work has shed considerable light on the epigenetic basis for var gene activation and silencing, how switching is controlled remains a mystery. In particular, switching seems not to be random, but instead appears to be coordinated to result in timely activation of individual genes leading to sequential waves of antigenically distinct parasite populations. The molecular basis for this apparent coordination is unknown. Here we show that var2csa, an unusual and highly conserved var gene, occupies a unique position within the var gene switching hierarchy. Induction of switching through the destabilization of var specific chromatin using both genetic and chemical methods repeatedly led to the rapid and exclusive activation of var2csa. Additional experiments demonstrated that these represent "true" switching events and not simply de-silencing of the var2csa promoter, and that activation is limited to the unique locus on chromosome 12. Combined with translational repression of var2csa transcripts, frequent "default" switching to this locus and detection of var2csa untranslated transcripts in non-pregnant individuals, these data suggest that var2csa could play a central role in coordinating switching, fulfilling a prediction made by mathematical models derived from population switching patterns. These studies provide the first insights into the mechanisms by which var gene switching is coordinated as well as an example of how a pharmacological agent can disrupt antigenic variation in Plasmodium falciparum.
Protein lysine methyltransferase G9a inhibitors: design, synthesis, and structure activity relationships of 2,4-diamino-7-aminoalkoxy-quinazolines.[Pubmed:20614940]
J Med Chem. 2010 Aug 12;53(15):5844-57.
Protein lysine methyltransferase G9a, which catalyzes methylation of lysine 9 of histone H3 (H3K9) and lysine 373 (K373) of p53, is overexpressed in human cancers. Genetic knockdown of G9a inhibits cancer cell growth, and the dimethylation of p53 K373 results in the inactivation of p53. Initial SAR exploration of the 2,4-diamino-6,7-dimethoxyquinazoline template represented by 3a (BIX01294), a selective small molecule inhibitor of G9a and GLP, led to the discovery of 10 (UNC0224) as a potent G9a inhibitor with excellent selectivity. A high resolution X-ray crystal structure of the G9a-10 complex, the first cocrystal structure of G9a with a small molecule inhibitor, was obtained. On the basis of the structural insights revealed by this cocrystal structure, optimization of the 7-dimethylaminopropoxy side chain of 10 resulted in the discovery of 29 (UNC0321) (Morrison K(i) = 63 pM), which is the first G9a inhibitor with picomolar potency and the most potent G9a inhibitor to date.


