Kazinol UCAS# 1238116-48-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1238116-48-7 | SDF | Download SDF |
| PubChem ID | 52316406 | Appearance | Powder |
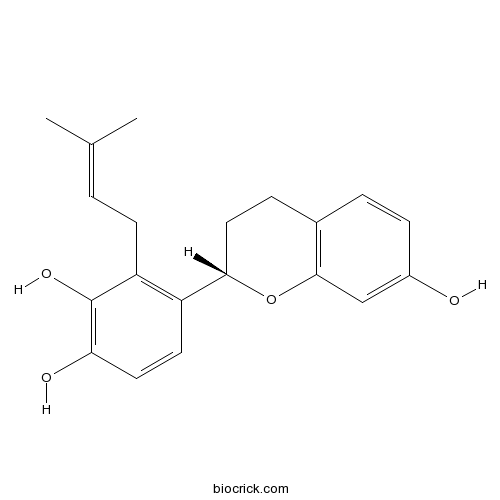
| Formula | C20H22O4 | M.Wt | 326.39 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(2S)-7-hydroxy-3,4-dihydro-2H-chromen-2-yl]-3-(3-methylbut-2-enyl)benzene-1,2-diol | ||
| SMILES | CC(=CCC1=C(C=CC(=C1O)O)C2CCC3=C(O2)C=C(C=C3)O)C | ||
| Standard InChIKey | MVHAAGZZSATGDD-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C20H22O4/c1-12(2)3-7-16-15(8-9-17(22)20(16)23)18-10-5-13-4-6-14(21)11-19(13)24-18/h3-4,6,8-9,11,18,21-23H,5,7,10H2,1-2H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kazinol U may have therapeutic value in delaying pancreatic β-cell destruction in type 1 diabetes by blocking the NF-κB pathway in pancreatic β-cells reduces cell damage. 2. Kazinol U shows estrogenic activity with ligand-activity of estrogen receptor, transcriptional activity of estrogen -responsive element-reporter genes, it may have beneficial effects in the treatment of menopausal symptoms. |
| Targets | Estrogen receptor | NF-kB | IkB | NO | NOS | IFN-γ | IL Receptor | IKK | Progestogen receptor |

Kazinol U Dilution Calculator

Kazinol U Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0638 mL | 15.3191 mL | 30.6382 mL | 61.2764 mL | 76.5955 mL |
| 5 mM | 0.6128 mL | 3.0638 mL | 6.1276 mL | 12.2553 mL | 15.3191 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0638 mL | 6.1276 mL | 7.6595 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6128 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6128 mL | 0.766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hopeachinol B
Catalog No.:BCN3445
CAS No.:1238083-45-8
- Hydroxyevodiamine
Catalog No.:BCN2491
CAS No.:1238-43-3
- QNZ 46
Catalog No.:BCC6292
CAS No.:1237744-13-6
- ML 786 dihydrochloride
Catalog No.:BCC7997
CAS No.:1237536-18-3
- Escin IA
Catalog No.:BCN3862
CAS No.:123748-68-5
- Aucherine
Catalog No.:BCN2058
CAS No.:123715-12-8
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- Moracin O
Catalog No.:BCN4004
CAS No.:123702-97-6
- Kuwanol C
Catalog No.:BCN3941
CAS No.:123702-94-3
- CGP 36216 hydrochloride
Catalog No.:BCC7605
CAS No.:123691-29-2
- CGP 46381
Catalog No.:BCC6990
CAS No.:123691-14-5
- CGP 35348
Catalog No.:BCC6988
CAS No.:123690-79-9
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
A prenylated flavan from Broussonetia kazinoki prevents cytokine-induced beta-cell death through suppression of nuclear factor-kappaB activity.[Pubmed:21720008]
Biol Pharm Bull. 2011;34(7):1026-31.
The generation of nitric oxide (NO) via inducible NO synthase (iNOS) and reactive oxygen species plays a key role in cytokine-mediated pancreatic beta-cell damage. Oxidative stress due to reactive oxygen species activates the nuclear factor-kappaB (NF-kappaB) transcription factor, which regulates iNOS expression. In this regard, suppression of the NF-kappaB pathway is a novel strategy for protecting beta-cells from damage. This study was performed to explore the effects of Kazinol U, a prenylated flavan from Broussonetia kazinoki, on the NF-kappaB activation pathway in interleukin-1beta (IL-1beta)- and interferon-gamma (IFN-gamma)-treated beta-cells. The cytotoxic effects of cytokines were completely abolished when RINm5F cells or islets were pretreated with Kazinol U. Kazinol U inhibited the nuclear translocation and DNA binding of NF-kappaB subunits, which correlated with the inhibitory effects on IkappaB kinase (IKK) phosphorylation and IkappaBalpha degradation. In addition, Kazinol U suppressed NO and hydrogen peroxide production and apoptotic cell death by cytokines in RINm5F cells. The protective effects of Kazinol U were further demonstrated by normal insulin secretion of cytokine-treated islets in response to glucose. Taken together, these results suggest that using Kazinol U to block the NF-kappaB pathway in pancreatic beta-cells reduces cell damage. Therefore, Kazinol U may have therapeutic value in delaying pancreatic beta-cell destruction in type 1 diabetes.
New estrogenic compounds isolated from Broussonetia kazinoki.[Pubmed:20493686]
Bioorg Med Chem Lett. 2010 Jun 15;20(12):3764-7.
Two new and two known compounds were identified as estrogenic constituents from Broussonetia kazinoki. Their structures were elucidated as broussonin A (1), tupichinol C (2), Kazinol U (3), and (+)-(2R) kazinol I (4). They showed estrogenic activity with ligand-binding activity of estrogen receptor, transcriptional activity of estrogen-responsive element-luciferase reporter genes. They also control the cellular gene expression levels of estrogen-responsive genes. Phytoestrogens from B. kazinoki may have beneficial effects in the treatment of menopausal symptoms.


