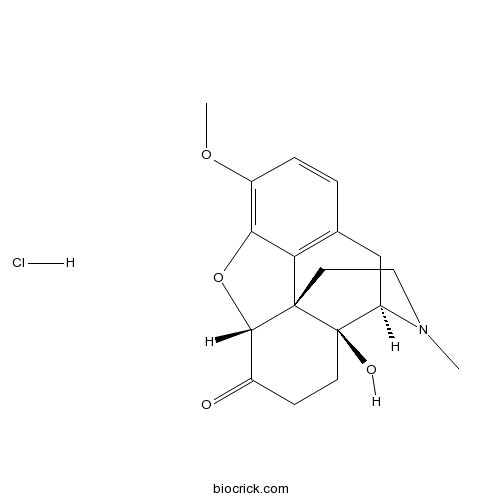
Oxycodone hydrochlorideCAS# 124-90-3 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124-90-3 | SDF | Download SDF |
| PubChem ID | 5462350 | Appearance | Powder |
| Formula | C18H22ClNO4 | M.Wt | 351.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (4R,4aS,7aR,12bS)-4a-hydroxy-9-methoxy-3-methyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride | ||
| SMILES | CN1CCC23C4C(=O)CCC2(C1CC5=C3C(=C(C=C5)OC)O4)O.Cl | ||
| Standard InChIKey | MUZQPDBAOYKNLO-RKXJKUSZSA-N | ||
| Standard InChI | InChI=1S/C18H21NO4.ClH/c1-19-8-7-17-14-10-3-4-12(22-2)15(14)23-16(17)11(20)5-6-18(17,21)13(19)9-10;/h3-4,13,16,21H,5-9H2,1-2H3;1H/t13-,16+,17+,18-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective μ-opioid receptor agonist (Ki values are 16 and >1000 nM for hMOR1 and hKOR1 respectively). |

Oxycodone hydrochloride Dilution Calculator

Oxycodone hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8424 mL | 14.2118 mL | 28.4236 mL | 56.8473 mL | 71.0591 mL |
| 5 mM | 0.5685 mL | 2.8424 mL | 5.6847 mL | 11.3695 mL | 14.2118 mL |
| 10 mM | 0.2842 mL | 1.4212 mL | 2.8424 mL | 5.6847 mL | 7.1059 mL |
| 50 mM | 0.0568 mL | 0.2842 mL | 0.5685 mL | 1.1369 mL | 1.4212 mL |
| 100 mM | 0.0284 mL | 0.1421 mL | 0.2842 mL | 0.5685 mL | 0.7106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- Kazinol U
Catalog No.:BCN4720
CAS No.:1238116-48-7
- Hopeachinol B
Catalog No.:BCN3445
CAS No.:1238083-45-8
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
- Kobophenol A
Catalog No.:BCN3444
CAS No.:124027-58-3
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
- 1-Caffeoylquinic acid
Catalog No.:BCN5911
CAS No.:1241-87-8
- 2-Hydroxytetracosanoic acid ethyl ester
Catalog No.:BCN1599
CAS No.:124111-47-3
- Scutebarbatine O
Catalog No.:BCN8377
CAS No.:960302-88-9
- Alcesefoliside
Catalog No.:BCN2933
CAS No.:124151-38-8
Premedication with fast-acting oxycodone hydrochloride hydrate effectively reduced oxaliplatin-induced severe vascular pain.[Pubmed:28285948]
J Infect Chemother. 2017 Jul;23(7):493-497.
Oxaliplatin is a platinum-based chemotherapeutic agent that holds a prominent position in the treatment of colorectal and gastric cancers. However, severe oxaliplatin-related vascular pain can be problematic for patients. Here we describe seven patients who experienced severe vascular pain caused by oxaliplatin administration. All seven patients were treated with capecitabine and oxaliplatin or capecitabine plus oxaliplatin with bevacizumab as an adjuvant or a treatment for recurrent colorectal cancer, respectively. Patients experienced intolerable vascular pain during oxaliplatin administration, which continued for several days. Moreover, vascular pain also induced insomnia and appetite loss in all patients. We recommended implantation of a central venous (CV) port to the patients; however, all patients declined this treatment. In addition, various known countermeasures were taken, but were ineffective. Therefore, patients were orally administered Oxycodone hydrochloride hydrate (Oxinorm((R))) 45 min prior to oxaliplatin administration. This pretreatment successfully reduced vascular pain and improved subsequent chemotherapy. Oxinorm((R)) is a fast-acting opioid that can be an effective and practical option for severe vascular pain induced by oxaliplatin. The present report is the first description that emphasizes the usefulness of Oxinorm((R)) to overcome the vascular pain induced by administration of oxaliplatin via a peripheral vein.
Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites.[Pubmed:16678548]
Clin Pharmacol Ther. 2006 May;79(5):461-79.
BACKGROUND: In vitro experiments suggest that circulating metabolites of oxycodone are opioid receptor agonists. Clinical and animal studies to date have failed to demonstrate a significant contribution of the O-demethylated metabolite oxymorphone toward the clinical effects of the parent drug, but the role of other putative circulating active metabolites in oxycodone pharmacodynamics remains to be examined. METHODS: Pharmacokinetics and pharmacodynamics of oxycodone were investigated in healthy human volunteers; measurements included the time course of plasma concentrations and urinary excretion of metabolites derived from N-demethylation, O-demethylation, and 6-keto-reduction, along with the time course of miosis and subjective opioid side effects. The contribution of circulating metabolites to oxycodone pharmacodynamics was analyzed by pharmacokinetic-pharmacodynamic modeling. The human study was complemented by in vitro measurements of opioid receptor binding and activation studies, as well as in vivo studies of the brain distribution of oxycodone and its metabolites in rats. RESULTS: Urinary metabolites derived from cytochrome P450 (CYP) 3A-mediated N-demethylation of oxycodone (noroxycodone, noroxymorphone, and alpha- and beta-noroxycodol) accounted for 45% +/- 21% of the dose, whereas CYP2D6-mediated O-demethylation (oxymorphone and alpha- and beta-oxymorphol) and 6-keto-reduction (alpha- and beta-oxycodol) accounted for 11% +/- 6% and 8% +/- 6% of the dose, respectively. Noroxycodone and noroxymorphone were the major metabolites in circulation with elimination half-lives longer than that of oxycodone, but their uptake into the rat brain was significantly lower compared with that of the parent drug. Pharmacokinetic-pharmacodynamic modeling indicated that the time course of pupil constriction is fully explained by the plasma concentration of the parent drug, oxycodone, alone. The metabolites do not contribute to the central effects, either because of their low potency or low abundance in circulation or as a result of their poor uptake into the brain. CONCLUSIONS: CYP3A-mediated N-demethylation is the principal metabolic pathway of oxycodone in humans. The central opioid effects of oxycodone are governed by the parent drug, with a negligible contribution from its circulating oxidative and reductive metabolites.


