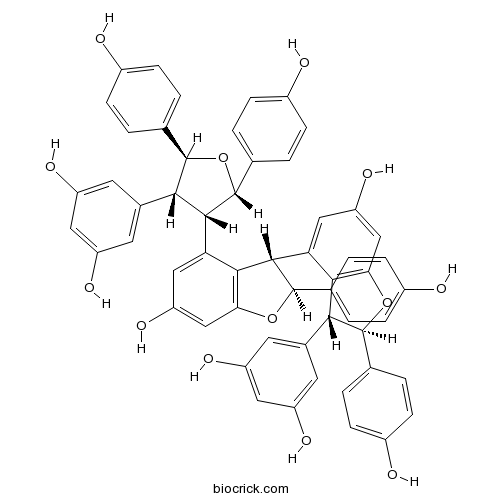
Kobophenol ACAS# 124027-58-3 |
- Carasinol B
Catalog No.:BCN8226
CAS No.:777857-86-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124027-58-3 | SDF | Download SDF |
| PubChem ID | 484758 | Appearance | Powder |
| Formula | C56H44O13 | M.Wt | 925.0 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[(2S,3R,4S,5S)-4-[(2S,3S)-3-[(2R,3R)-3-(3,5-dihydroxyphenyl)-6-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-4-yl]-6-hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-4-yl]-2,5-bis(4-hydroxyphenyl)oxolan-3-yl]benzene-1,3-diol | ||
| SMILES | C1=CC(=CC=C1C2C(C(C(O2)C3=CC=C(C=C3)O)C4=CC(=CC5=C4C(C(O5)C6=CC=C(C=C6)O)C7=CC(=CC8=C7C(C(O8)C9=CC=C(C=C9)O)C1=CC(=CC(=C1)O)O)O)O)C1=CC(=CC(=C1)O)O)O | ||
| Standard InChIKey | RAUCCLKIJHMTND-LUPMIFTGSA-N | ||
| Standard InChI | InChI=1S/C56H44O13/c57-33-9-1-27(2-10-33)53-47(31-17-37(61)21-38(62)18-31)49-43(23-41(65)25-45(49)67-53)52-50-44(24-42(66)26-46(50)68-55(52)29-5-13-35(59)14-6-29)51-48(32-19-39(63)22-40(64)20-32)54(28-3-11-34(58)12-4-28)69-56(51)30-7-15-36(60)16-8-30/h1-26,47-48,51-66H/t47-,48+,51-,52+,53+,54-,55-,56-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Kobophenol A has antimicrobial, and anti-inflammatory activities, it might be a candidate for treatment of inflammatory bone diseases relevant to osteoblast cell death. Kobophenol A inhibits AChE activity in a dose-dependent manner, and the IC50 value is 115.8mM. Kobophenol A has protective effect against nitrosative/oxidative or mitochondrial damages resulted in the inhibition of the ROS, intracellular calcium ion level, and mitochondrial transmembrane potential changes on SH-SY5Y cells. |
| Targets | p38MAPK | CDK | ROS | Bcl-2/Bax | JNK | AP-1 | NO | NF-kB | MMP(e.g.TIMP) |
| In vitro | Kobophenol A enhances proliferation of human osteoblast-like cells with activation of the p38 pathway.[Pubmed: 24021754]Int Immunopharmacol. 2013 Nov;17(3):704-13.Bone cell proliferation, bone formation, and bone resorption are the main factors involved in the homeostasis of the bone mass. Osteoblast death is a problem experienced by postmenopause women. Herbal medicines have attracted considerable attention for use as a drug or a drug substitute in the treatment of bone-related diseases, such as osteoporosis. Kobophenol A inhibits sodium nitroprusside-induced cardiac H9c2 cell death through suppressing activation of JNK and preserving mitochondrial anti-apoptotic Bcl-2 and Mcl-1.[Pubmed: 24759620]Chem Pharm Bull (Tokyo). 2014;62(7):713-8. Epub 2014 Apr 24.Sodium nitroprusside (SNP) releases nitric oxide (NO), a powerful vasodilator, and thus widely used in intensive care unit for treating hypertension emergency. However, cardiac toxicity after SNP administration is a clinical problem. |
| Kinase Assay | Neuroprotective effects of kobophenol A against the withdrawal of tropic support, nitrosative stress, and mitochondrial damage in SH-SY5Y neuroblastoma cells.[Pubmed: 17300930]Bioorg Med Chem Lett. 2007 Apr 1;17(7):1879-82. Epub 2007 Jan 27.This study examined the neuroprotective effects of Kobophenol A (kob A), oligomeric stillbene, and a resveratrol tetramer. Neuronal death induced by the withdrawal of tropic support was ameliorated by Kobophenol A. The protective effect of Kobophenol A against nitrosative/oxidative or mitochondrial damages resulted in the inhibition of the ROS, intracellular calcium ion level, and mitochondrial transmembrane potential changes on SH-SY5Y cells. |
| Cell Research | Protective effect of kobophenol A on nitric oxide-induced cell apoptosis in human osteoblast-like MG-63 cells: involvement of JNK, NF-κB and AP-1 pathways.[Pubmed: 21511059]Int Immunopharmacol. 2011 Sep;11(9):1251-9.Nitric oxide (NO) is a multifunctional signaling molecule and the cytotoxic species responsible for a variety of pathologic disorders including bone destruction. High NO levels induce the apoptosis of osteoblasts and decrease the bone mineral density. |

Kobophenol A Dilution Calculator

Kobophenol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0811 mL | 5.4054 mL | 10.8108 mL | 21.6216 mL | 27.027 mL |
| 5 mM | 0.2162 mL | 1.0811 mL | 2.1622 mL | 4.3243 mL | 5.4054 mL |
| 10 mM | 0.1081 mL | 0.5405 mL | 1.0811 mL | 2.1622 mL | 2.7027 mL |
| 50 mM | 0.0216 mL | 0.1081 mL | 0.2162 mL | 0.4324 mL | 0.5405 mL |
| 100 mM | 0.0108 mL | 0.0541 mL | 0.1081 mL | 0.2162 mL | 0.2703 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
- 1-Caffeoylquinic acid
Catalog No.:BCN5911
CAS No.:1241-87-8
- 2-Hydroxytetracosanoic acid ethyl ester
Catalog No.:BCN1599
CAS No.:124111-47-3
- Scutebarbatine O
Catalog No.:BCN8377
CAS No.:960302-88-9
- Alcesefoliside
Catalog No.:BCN2933
CAS No.:124151-38-8
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
- 6-O-Vanilloylajugol
Catalog No.:BCN6125
CAS No.:124168-04-3
- Laxiracemosin H
Catalog No.:BCN6910
CAS No.:1241871-28-2
Neuroprotective effects of kobophenol A against the withdrawal of tropic support, nitrosative stress, and mitochondrial damage in SH-SY5Y neuroblastoma cells.[Pubmed:17300930]
Bioorg Med Chem Lett. 2007 Apr 1;17(7):1879-82.
This study examined the neuroprotective effects of Kobophenol A (kob A), oligomeric stillbene, and a resveratrol tetramer. Neuronal death induced by the withdrawal of tropic support was ameliorated by kob A. The protective effect of kob A against nitrosative/oxidative or mitochondrial damages resulted in the inhibition of the ROS, intracellular calcium ion level, and mitochondrial transmembrane potential changes on SH-SY5Y cells.
Kobophenol A enhances proliferation of human osteoblast-like cells with activation of the p38 pathway.[Pubmed:24021754]
Int Immunopharmacol. 2013 Nov;17(3):704-13.
Bone cell proliferation, bone formation, and bone resorption are the main factors involved in the homeostasis of the bone mass. Osteoblast death is a problem experienced by postmenopause women. Herbal medicines have attracted considerable attention for use as a drug or a drug substitute in the treatment of bone-related diseases, such as osteoporosis. This study investigated the effects of Kobophenol A on the proliferation in human osteoblast cells. Kobophenol A stimulated the proliferation of osteoblast cells by the increases in DNA synthesis and the enhancement of cell cycle progression. Kobophenol A stimulation induced the expression of the cyclin B1 and cyclin-dependent kinase 1 (CDK1). Treatment of osteoblast cells with p38 MAPK inhibitor SB203580 significantly inhibited Kobophenol A-enhanced proliferation. In addition, Kobophenol A induced phosphorylation of p38 MAPK. Treatment of osteoblast cells with Kobophenol A resulted in improvement of ROS scavenging activity. Moreover, Kobophenol A treatment up-regulated the Bcl-2 level, but down-regulated the level of Bax expression. We also demonstrate that Kobophenol A increased alkaline phosphatase (ALP) activity after 2 days. Taken together, the results of this study reveal that Kobophenol A has proliferative effects and enhances ALP activity in osteoblast cells and these findings provide insights into the development of a therapeutic approach of Kobophenol A in the prevention of osteoporosis and other bone disorders.
Protective effect of kobophenol A on nitric oxide-induced cell apoptosis in human osteoblast-like MG-63 cells: involvement of JNK, NF-kappaB and AP-1 pathways.[Pubmed:21511059]
Int Immunopharmacol. 2011 Sep;11(9):1251-9.
Nitric oxide (NO) is a multifunctional signaling molecule and the cytotoxic species responsible for a variety of pathologic disorders including bone destruction. High NO levels induce the apoptosis of osteoblasts and decrease the bone mineral density. We investigated the influence of Kobophenol A (kob A) on apoptosis in cultured human osteoblast-like MG-63 cells. Direct NO donor sodium nitroprusside (SNP) that has been recognized as an inducer of apoptosis in various cell lines significantly induced cell death and NO production in MG-63 cells. Coincubation of kob A in SNP-treated MG-63 cells resulted in a significant protection against NO-induced cell death. This is associated with increase in intracellular reactive oxygen species (ROS) scavenging activity and the inhibition of decrease in mitochondrial membrane potential (MMP) by kob A. We also found that kob A inhibited the down-regulation of Bcl-2 and Bcl-X(L), whereas the level of Bax expression was decreased by kob A treatment in SNP-treated MG-63 cells. Furthermore, kob A inhibited SNP-induced phosphorylation of JNK and c-Jun, and SNP-induced reduction in NF-kappaBeta and AP-1 activities, implicating that protective effect of kob A may occur through the regulation of JNK, NF-kappaBeta and AP-1 signaling pathways. Together, these findings suggest that kob A has a protective effect against NO-mediated osteoblast apoptosis and might be a plausible candidate for treatment of inflammatory bone diseases relevant to osteoblast cell death.
Kobophenol A inhibits sodium nitroprusside-induced cardiac H9c2 cell death through suppressing activation of JNK and preserving mitochondrial anti-apoptotic Bcl-2 and Mcl-1.[Pubmed:24759620]
Chem Pharm Bull (Tokyo). 2014;62(7):713-8. Epub 2014 Apr 24.
Sodium nitroprusside (SNP) releases nitric oxide (NO), a powerful vasodilator, and thus widely used in intensive care unit for treating hypertension emergency. However, cardiac toxicity after SNP administration is a clinical problem. For finding a natural compound that suppressing SNP-induced cardiac toxicity, we tested the protective potential of Kobophenol A (Kob A), purified from the root of Caragana sinica, against the toxic effects of SNP. The severe cardiac H9c2 cell death was induced by SNP (2 mM) treatment. Kob A ameliorated SNP-induced cardiac H9c2 cell death, and this protective effect of Kob A may be related to the inhibition of c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase activation following SNP administration. In addition, the downregulation of cellular Bcl-2 and Mcl-1 levels by SNP exposure was strongly abrogated in the presence of Kob A. These biological properties of Kob A might provide insights into developing new cardioprotectant against SNP-induced cardiac cell death.
Formation of a new oxidative metabolite from kobophenol A by human intestinal bacterium Klebsiella pneumoniae.[Pubmed:17191999]
Chem Biodivers. 2005 Apr;2(4):506-9.
During our studies on the metabolism of Kobophenol A (1) in rats, we had isolated, purified, and identified the main new oxidative metabolite of 1, koboquinone A (2) from rats' feces. To elucidate the metabolic pathway of 1 in rats, we conducted the in vitro metabolic experiments of 1 by human intestinal bacteria and found that Klebsiella pneumoniae produced appreciable amounts of 2. This was verified by means of high performance liquid chromatography mass/mass spectrometry (HPLC/MS/MS) analysis.


