1beta,10beta-EpoxydesacetoxymatricarinCAS# 124020-39-9 |
Quality Control & MSDS
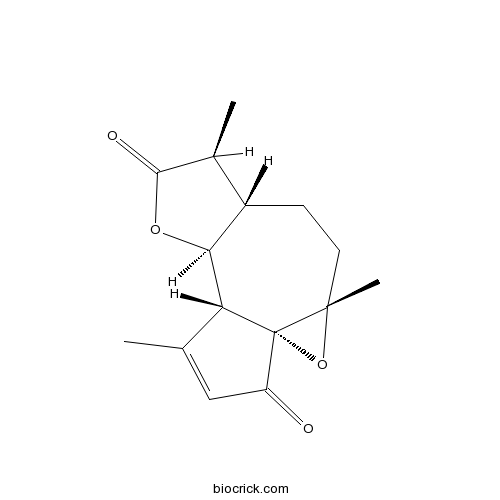
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 124020-39-9 | SDF | Download SDF |
| PubChem ID | 14313751 | Appearance | Powder |
| Formula | C15H18O4 | M.Wt | 262.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,3S,6S,7S,10S,11R)-3,7,12-trimethyl-2,9-dioxatetracyclo[9.3.0.01,3.06,10]tetradec-12-ene-8,14-dione | ||
| SMILES | CC1C2CCC3(C4(O3)C(C2OC1=O)C(=CC4=O)C)C | ||
| Standard InChIKey | WLWQUBMOSXUFJB-YFRBHOLXSA-N | ||
| Standard InChI | InChI=1S/C15H18O4/c1-7-6-10(16)15-11(7)12-9(8(2)13(17)18-12)4-5-14(15,3)19-15/h6,8-9,11-12H,4-5H2,1-3H3/t8-,9-,11+,12-,14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1beta,10beta-Epoxydesacetoxymatricarin shows high anti-hypercholesterolemic potential. |
| Targets | HMG-CoA Reductase | TNF-α | MMP(e.g.TIMP) |

1beta,10beta-Epoxydesacetoxymatricarin Dilution Calculator

1beta,10beta-Epoxydesacetoxymatricarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8124 mL | 19.0621 mL | 38.1243 mL | 76.2486 mL | 95.3107 mL |
| 5 mM | 0.7625 mL | 3.8124 mL | 7.6249 mL | 15.2497 mL | 19.0621 mL |
| 10 mM | 0.3812 mL | 1.9062 mL | 3.8124 mL | 7.6249 mL | 9.5311 mL |
| 50 mM | 0.0762 mL | 0.3812 mL | 0.7625 mL | 1.525 mL | 1.9062 mL |
| 100 mM | 0.0381 mL | 0.1906 mL | 0.3812 mL | 0.7625 mL | 0.9531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- Topotecan
Catalog No.:BCC5646
CAS No.:123948-87-8
- Hypocrellin B
Catalog No.:BCN3397
CAS No.:123940-54-5
- (R)-(+)-HA-966
Catalog No.:BCC6588
CAS No.:123931-04-4
- Cassiaside
Catalog No.:BCN2939
CAS No.:123914-49-8
- Gentiside J
Catalog No.:BCN7306
CAS No.:1238837-50-7
- PCA 4248
Catalog No.:BCC6699
CAS No.:123875-01-4
- UNC0321
Catalog No.:BCC4142
CAS No.:1238673-32-9
- Kobophenol A
Catalog No.:BCN3444
CAS No.:124027-58-3
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
- 1-Caffeoylquinic acid
Catalog No.:BCN5911
CAS No.:1241-87-8
- 2-Hydroxytetracosanoic acid ethyl ester
Catalog No.:BCN1599
CAS No.:124111-47-3
- Scutebarbatine O
Catalog No.:BCN8377
CAS No.:960302-88-9
- Alcesefoliside
Catalog No.:BCN2933
CAS No.:124151-38-8
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
- 6-O-Vanilloylajugol
Catalog No.:BCN6125
CAS No.:124168-04-3
Intermolecular exciton coupling and vibronic effects in solid-state circular dichroism: a case study.[Pubmed:23203006]
Phys Chem Chem Phys. 2013 Jan 21;15(3):795-802.
The electronic circular dichroism (CD) spectrum of the sesquiterpenoid 1beta,10beta-Epoxydesacetoxymatricarin (1) measured in the microcrystalline solid state differs from the solution one in the appearance of a pronounced vibrational fine structure in the long-wavelength region (n-pi* enone transition) and of a new moderately intense band in the pi-pi* region. TDDFT CD calculations were run on input structures derived from the X-ray geometry of 1 to reproduce the impact of exciton-type couplings between proximate molecules in the crystals. The vibrational structure of the CD spectrum was also reproduced for the isolated molecule by modelling the potential energy surfaces at the harmonic level and taking into account Duschinsky and Herzberg-Teller effects.
The Chemical Composition of Achillea wilhelmsii C. Koch and Its Desirable Effects on Hyperglycemia, Inflammatory Mediators and Hypercholesterolemia as Risk Factors for Cardiometabolic Disease.[Pubmed:27023504]
Molecules. 2016 Mar 25;21(4):404.
This study was done to identify the content compounds of Achillea wilhelmsii (A. wilhelmsii) and to evaluate its hypoglycemic and anti-hypercholesterolemic activity and effect on inflammatory mediators. The extracts and fractions of A. wilhelmsii were thoroughly analyzed using high performance liquid chromatography (HPLC), and the total content of phenols and flavonoids was determined. The hypoglycemic activity was evaluated in vivo using alloxan-induced diabetic mice. The effect upon inflammatory mediators was evaluated in vitro using the human monocytic leukemia cell line (THP-1). The anti-hypercholesterolemic activity was evaluated in vitro using the 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase assay kit. The water extract (WE)-treated group showed the highest reduction in the fasting blood glucose levels (FBGL). The chloroform fraction (CF) and ethyl acetate fraction (EAF) both showed a significant ability to reduce the secretion of tumor necrosis factor alpha (TNF-alpha). The EAF, however, also attenuated the levels of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9). The CF showed the most significant 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) inhibition activity. The five main compounds in the CF were isolated and identified. Out of the five compounds in the CF, 1beta,10beta-Epoxydesacetoxymatricarin (CP1) and leucodin (CP2) showed the highest anti-hypercholesterolemic potential. A molecular docking study provided corresponding results.


