MussaenosideCAS# 64421-27-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64421-27-8 | SDF | Download SDF |
| PubChem ID | 182423 | Appearance | Powder |
| Formula | C17H26O10 | M.Wt | 390.4 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
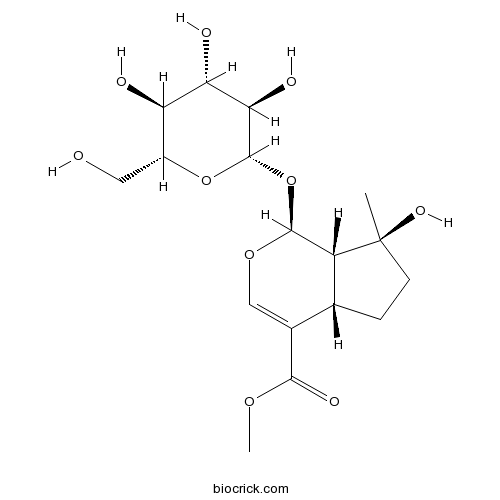
| Chemical Name | methyl (1S,4aS,7S,7aS)-7-hydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,5,6,7a-tetrahydro-1H-cyclopenta[c]pyran-4-carboxylate | ||
| SMILES | CC1(CCC2C1C(OC=C2C(=O)OC)OC3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | XBGJTRDIWPEIMG-DUMNYRKASA-N | ||
| Standard InChI | InChI=1S/C17H26O10/c1-17(23)4-3-7-8(14(22)24-2)6-25-15(10(7)17)27-16-13(21)12(20)11(19)9(5-18)26-16/h6-7,9-13,15-16,18-21,23H,3-5H2,1-2H3/t7-,9-,10-,11-,12+,13-,15+,16+,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Mussaenoside has anti-inflammatory action, the mechanism associated with downregulation of nuclear factor-κB. 2. Mussaenoside can inhibit the release of pro-inflammatory cytokines induced by LPS, the production of nitric oxide (NO) and prostaglandin E2, and the expression of inducible NO synthase and cyclooxygenase-2 induced by lipopolysaccharide (LPS) in the RAW264.7 murine macrophage cell line. |
| Targets | TNF-α | IL Receptor | NF-kB | NO | PGE | NOS |

Mussaenoside Dilution Calculator

Mussaenoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5615 mL | 12.8074 mL | 25.6148 mL | 51.2295 mL | 64.0369 mL |
| 5 mM | 0.5123 mL | 2.5615 mL | 5.123 mL | 10.2459 mL | 12.8074 mL |
| 10 mM | 0.2561 mL | 1.2807 mL | 2.5615 mL | 5.123 mL | 6.4037 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5123 mL | 1.0246 mL | 1.2807 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2561 mL | 0.5123 mL | 0.6404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DL-2-Amino-n-octanoic acid
Catalog No.:BCC8949
CAS No.:644-90-6
- Precocene II
Catalog No.:BCN4605
CAS No.:644-06-4
- Alloalantolactone
Catalog No.:BCN8091
CAS No.:64340-41-6
- N6-Benzoyl-5'-O-(4,4'-dimethoxytrityl)-2'-deoxyadenosine
Catalog No.:BCC9074
CAS No.:64325-78-6
- Malvidin chloride
Catalog No.:BCN3017
CAS No.:643-84-5
- Dracorhodin
Catalog No.:BCC9226
CAS No.:643-56-1
- Galactopinitol A
Catalog No.:BCC8926
CAS No.:64290-91-1
- Acetagastrodin
Catalog No.:BCN8155
CAS No.:64291-41-4
- Tetrahydropapaverine HCl
Catalog No.:BCC5321
CAS No.:6429-04-5
- Kielcorin
Catalog No.:BCN7637
CAS No.:64280-48-4
- Boc-N-Me-Tyr(Bzl)-OH
Catalog No.:BCC3356
CAS No.:64263-81-6
- Glutinol acetate
Catalog No.:BCN6675
CAS No.:6426-44-4
- Shanzhiside methylester
Catalog No.:BCN4187
CAS No.:64421-28-9
- Grifolin monomethyl ether
Catalog No.:BCN7568
CAS No.:64432-04-8
- Dulcoside A
Catalog No.:BCN3237
CAS No.:64432-06-0
- 10-Hydroxycamptothecin
Catalog No.:BCN1226
CAS No.:64439-81-2
- Tizanidine HCl
Catalog No.:BCC4357
CAS No.:64461-82-1
- Picroside III
Catalog No.:BCN6324
CAS No.:64461-95-6
- Glycoursodeoxycholic acid
Catalog No.:BCN7369
CAS No.:64480-66-6
- Cefotaxime sodium
Catalog No.:BCC8908
CAS No.:64485-93-4
- Rubianthraquinone
Catalog No.:BCN6880
CAS No.:644967-44-2
- Altretamine
Catalog No.:BCC1216
CAS No.:645-05-6
- Isovanillic acid
Catalog No.:BCN3376
CAS No.:645-08-9
- Scoulerine
Catalog No.:BCN6623
CAS No.:6451-73-6
Secondary metabolites isolated from Castilleja rubra exert anti-inflammatory effects through NF-kappaB inactivation on lipopolysaccharide-induced RAW264.7 macrophages.[Pubmed:24062082]
Arch Pharm Res. 2014 Jul;37(7):947-54.
8-Epiloganin (1), Mussaenoside (2), and 5-O-caffeoylshikimic acid (3) have been isolated from Castilleja rubra, and the anti-inflammatory properties of these metabolites in a cell culture system were investigated. Compounds 1-3 suppressed not only the production of nitric oxide (NO) and prostaglandin E2, but also the expression of inducible NO synthase and cyclooxygenase-2 induced by lipopolysaccharide (LPS) in the RAW264.7 murine macrophage cell line. Compounds 1-3 also inhibited the release of pro-inflammatory cytokines induced by LPS, namely, tumor necrosis factor-alpha and interleukin-1beta. The underlying mechanism of the anti-inflammatory action of compounds 1-3 was associated with downregulation of nuclear factor-kappaB.
The Herbal Drug Melampyrum pratense L. (Koch): Isolation and Identification of Its Bioactive Compounds Targeting Mediators of Inflammation.[Pubmed:23533479]
Evid Based Complement Alternat Med. 2013;2013:395316.
Melampyrum pratense L. (Koch) is used in traditional Austrian medicine for the treatment of different inflammation-related conditions. In this work, we show that the extracts of M. pratense stimulated peroxisome proliferator-activated receptors- (PPARs-) alpha and - gamma that are well recognized for their anti-inflammatory activities. Furthermore, the extract inhibited the activation of the proinflammatory transcription factor NF- kappa B and induction of its target genes interleukin-8 (IL-8) and E-selectin in vitro. Bioassay-guided fractionation identified several active flavonoids and iridoids including melampyroside and Mussaenoside and the phenolic compound lunularin that were identified in this species for the first time. The flavonoids apigenin and luteolin were distinguished as the main components accountable for the anti-inflammatory properties. Apigenin and luteolin effectively inhibited tumor necrosis factor alpha (TNF- alpha )-induced NF- kappa B-mediated transactivation of a luciferase reporter gene. Furthermore, the two compounds dose-dependently reduced IL-8 and E-selectin protein expression after stimulation with lipopolysaccharide (LPS) or TNF- alpha in endothelial cells (ECs). The iridoids melampyroside and Mussaenoside prevented the elevation of E-selectin in LPS-stimulated ECs. Lunularin was found to reduce the protein levels of the proinflammatory mediators E-selectin and IL-8 in ECs in response to LPS. These data validate the ethnomedical use of M. pratense for the treatment of inflammatory conditions and point to the constituents accountable for its anti-inflammatory activity.
Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora.[Pubmed:24084016]
Molecules. 2013 Sep 30;18(10):12109-18.
Castilleja tenuiflora (Orobanchaceae) has been used in Mexican traditional medicine as a treatment for cough, dysentery, anxiety, nausea and vomiting as well as hepatic and gastrointestinal diseases. The ethanolic extract of the aerial parts of Castilleja tenuiflora was separated by silica gel column chromatography. The fractions were evaluated using the induced edema acetate 12-O-tetradecanoylphorbol (TPA) anti-inflammatory activity model. The most active fraction was subjected to medium-pressure liquid chromatography (MPLC) with UV detection at 206 and 240 nm. The following iridoids were isolated: geniposidic acid, aucubin, bartioside, 8-epi-loganin, Mussaenoside, and the phenylpropanoid verbascoside. The most active iridoid was geniposidic acid, which was more active than the control (indomethacin), and the least active iridoid was Mussaenoside. 8-epi-Loganin, and Mussaenoside have not been previously reported to be anti-inflammatory compounds. The results of these investigations confirm the potential of Mexican plants for the production of bioactive compounds and validate the ethnomedical use of Castilleja tenuiflora-like anti-inflammatory plants.


