Neoechinulin ACAS# 51551-29-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51551-29-2 | SDF | Download SDF |
| PubChem ID | 9996305 | Appearance | Powder |
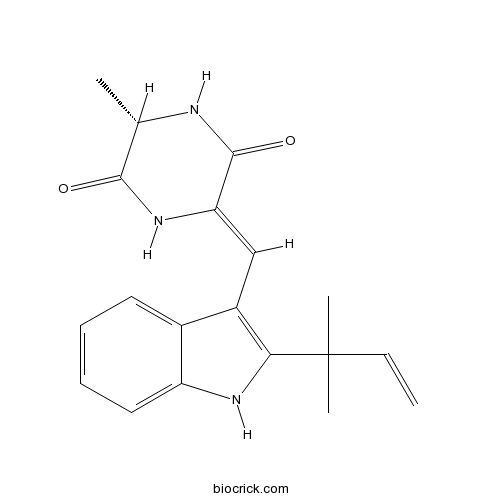
| Formula | C19H21N3O2 | M.Wt | 323.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,6Z)-3-methyl-6-[[2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl]methylidene]piperazine-2,5-dione | ||
| SMILES | CC1C(=O)NC(=CC2=C(NC3=CC=CC=C32)C(C)(C)C=C)C(=O)N1 | ||
| Standard InChIKey | MYRPIYZIAHOECW-SAIXKJTDSA-N | ||
| Standard InChI | InChI=1S/C19H21N3O2/c1-5-19(3,4)16-13(12-8-6-7-9-14(12)21-16)10-15-18(24)20-11(2)17(23)22-15/h5-11,21H,1H2,2-4H3,(H,20,24)(H,22,23)/b15-10-/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Neoechinulin A may block the phosphorylation of mitogen-activated protein kinase (MAPK) molecule p38, apoptosis signal-regulating kinase 1 (ASK-1) and nuclear translocation of nuclear factor-κB (NF-κB) p65 and p50 subunits. 2. Neoechinulin A has anti-inflammatory effect against LPS-stimulated RAW264.7 macrophages through inhibition of the NF-κB and p38 MAPK pathways. 3. Neoechinulin A may ameliorate rotenone toxicity by activating a cytoprotective machinery that requires ATP and antioxidant/anti-nitration activities. 4. Neoechinulin A has a potential to be developed as a modulator of neuroinflammatory process in Alzheimer's disease. |
| Targets | NO | PGE | NOS | COX | TNF-α | NF-kB | IkB | p38MAPK | Beta Amyloid | ASK | NADPH-oxidase | IKK |

Neoechinulin A Dilution Calculator

Neoechinulin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0921 mL | 15.4607 mL | 30.9215 mL | 61.8429 mL | 77.3036 mL |
| 5 mM | 0.6184 mL | 3.0921 mL | 6.1843 mL | 12.3686 mL | 15.4607 mL |
| 10 mM | 0.3092 mL | 1.5461 mL | 3.0921 mL | 6.1843 mL | 7.7304 mL |
| 50 mM | 0.0618 mL | 0.3092 mL | 0.6184 mL | 1.2369 mL | 1.5461 mL |
| 100 mM | 0.0309 mL | 0.1546 mL | 0.3092 mL | 0.6184 mL | 0.773 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Vitexin argininate
Catalog No.:BCC8179
CAS No.:51542-56-4
- GW 803430
Catalog No.:BCC7897
CAS No.:515141-51-2
- Adiantulupanone
Catalog No.:BCN7360
CAS No.:51511-05-8
- Cochinchinenin A
Catalog No.:BCN3496
CAS No.:221696-69-1
- (+)-Turicine
Catalog No.:BCC8361
CAS No.:515-24-2
- Sclareol
Catalog No.:BCN2395
CAS No.:515-03-7
- H-Tyr(tBu)-OMe.HCl
Catalog No.:BCC2672
CAS No.:51482-39-4
- PMX 205
Catalog No.:BCC8039
CAS No.:514814-49-4
- Cimetidine
Catalog No.:BCC4527
CAS No.:51481-61-9
- Deoxynivalenol
Catalog No.:BCC7832
CAS No.:51481-10-8
- Cyclo(Tyr-Phe)
Catalog No.:BCN2423
CAS No.:5147-17-1
- DMH4
Catalog No.:BCC6196
CAS No.:515880-75-8
- Cuspidiol
Catalog No.:BCN3942
CAS No.:51593-96-5
- Methylmalonate
Catalog No.:BCC7986
CAS No.:516-05-2
- Taurochenodeoxycholic Acid
Catalog No.:BCN8419
CAS No.:516-35-8
- Cerevisterol
Catalog No.:BCN5640
CAS No.:516-37-0
- Allopregnanolone
Catalog No.:BCC7737
CAS No.:516-54-1
- 20(S)-Hydroxycholesterol
Catalog No.:BCC7937
CAS No.:516-72-3
- Z-D-Glu(OtBu)-OH
Catalog No.:BCC2771
CAS No.:51644-83-8
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- Murrangatin diacetate
Catalog No.:BCN5641
CAS No.:51650-59-0
- 2-Methoxyphenalen-1-one
Catalog No.:BCN7181
CAS No.:51652-39-2
- Erythrartine
Catalog No.:BCN5642
CAS No.:51666-26-3
Neoechinulin a impedes the progression of rotenone-induced cytotoxicity in PC12 cells.[Pubmed:21415535]
Biol Pharm Bull. 2011;34(2):243-8.
Neoechinulin A, an indole alkaloid from marine fungi, can protect PC12 cells from the cytotoxicity of 1-methyl-4-phenylpyridinium (MPP(+)), a Parkinson disease-inducing neurotoxin, by ameliorating downstream events resulting from mitochondrial complex I inactivation. However, the cytoprotective mechanisms remained unclear. In this study, by using rotenone, another parkinsonian-inducing neurotoxin targeting mitochondrial complex I, we investigated the cytoprotective mechanism of Neoechinulin A. Rotenone-induced cell death was associated with accelerated glucose consumption, and excess glucose supplementation in the culture medium almost completely suppressed cell death, suggesting that glucose deficiency in the medium is critical for triggering cell death in this model. Co-treatment with Neoechinulin A, but not Neoechinulin A pre-treatment before rotenone exposure, significantly impeded cell death by rotenone. Although the presence of Neoechinulin A did not affect the accelerated glycolytic turnover in rotenone-treated cells, it paradoxically decreased ATP levels in the cells, suggesting increased ATP consumption. Although the link between the decreased ATP levels and cytoprotection is not clear at present, it suggests that Neoechinulin A may ameliorate rotenone toxicity by activating a cytoprotective machinery that requires ATP.
Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-small ka, CyrillicB and p38 MAPK Pathways in lipopolysaccharide-stimulated RAW264.7 macrophages.[Pubmed:24165583]
Molecules. 2013 Oct 25;18(11):13245-59.
In the course of a bioassay-guided study of metabolites from the marine fungus Eurotium sp. SF-5989, two diketopiperazine type indole alkaloids, neoechinulins A and B, were isolated. In this study, we investigated the anti-inflammatory effects of neoechinulins A (1) and B (2) on lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Neoechinulin A (1) markedly suppressed the production of nitric oxide (NO) and prostaglandin E2 (PGE2) and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in a dose dependent manner ranging from 12.5 microM to 100 microM without affecting the cell viability. On the other hand, neoechinulin B (2) affected the cell viability at 25 microM although the compound displayed similar inhibitory effect of NO production to Neoechinulin A (1) at lower doses. Furthermore, Neoechinulin A (1) decreased the secretion of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta). We also confirmed that Neoechinulin A (1) blocked the activation of nuclear factor-kappaB (NF-kappaB) in LPS-stimulated RAW264.7 macrophages by inhibiting the phosphorylation and degradation of inhibitor kappa B (IkappaB)-alpha. Moreover, Neoechinulin A (1) decreased p38 mitogen-activated protein kinase (MAPK) phosphorylation. Therefore, these data showed that the anti-inflammatory effects of Neoechinulin A (1) in LPS-stimulated RAW264.7 macrophages were due to the inhibition of the NF-kappaB and p38 MAPK pathways, suggesting that Neoechinulin A (1) might be a potential therapeutic agent for the treatment of various inflammatory diseases.
Neoechinulin a imparts resistance to acute nitrosative stress in PC12 cells: a potential link of an elevated cellular reserve capacity for pyridine nucleotide redox turnover with cytoprotection.[Pubmed:22791159]
Biol Pharm Bull. 2012;35(7):1105-17.
Treatment of PC12 cells with fungus-derived alkaloid Neoechinulin A for more than 12 h renders the cells resistant to subsequent superoxide (O(2)(-))/nitric oxide (NO) insults derived from 3-morpholinosydnonimine (SIN-1). However, the underlying mechanism(s) remains largely unclear. To elucidate the mechanism(s), we assessed the specificity of the cytoprotection afforded by Neoechinulin A treatment using other cytocidal stressors and also clarified the resulting cellular alterations, focusing on the antioxidant and metabolic enzymes systems. Neoechinulin A treatment for more than 12 h endowed PC12 cells with significant resistance to transient NO toxicity, but not persistent NO toxicity, bolus H(2)O(2) toxicity, or oxidative insult from the redox cycling quinone menadione. Cellular antioxidant system profiling revealed no substantial potentiation of the activity of any antioxidant enzyme in lysate from the Neoechinulin A-treated cells excluding glutathione (GSH) content, which was significantly decreased (>50%), resulting in a proportional compromise in the thiol-reducing activity of the intact cells. In addition, no differences were observed in the activity for any nicotinamide adenine dinucleotide (phosphate) reduced form (NAD(P)H)-generating enzyme, steady-state NAD(P)H/nicotinamide adenine dinucleotide (phosphate) oxidized form (NAD(P)(+)) ratios, or the levels of total NAD(P)H. Nevertheless, the Neoechinulin A-treated intact cells exhibited increased NAD(P)H redox turnover when driven by extracellular tetrazolium. The structurally inactive analog preechinulin failed to protect cells against NO toxicity or induce these alterations, suggesting their link with the cytoprotective mechanism. These results suggest that Neoechinulin A, despite disabling the GSH defense system, confers cytoprotection against nitrosative stresses by elevating the cellular reserve capacity for NAD(P)H generation, which could offset crippling of energy-supplying systems due to nitrosative stress.
Structure-activity relationships of neoechinulin A analogues with cytoprotection against peroxynitrite-induced PC12 cell death.[Pubmed:17965477]
J Antibiot (Tokyo). 2007 Oct;60(10):614-21.
Neoechinulin A, an alkaloid from Eurotium rubrum Hiji025, protected neuronal PC12 cells against cell death induced by peroxynitrite derived from SIN-1 (3-(4-morpholinyl)sydnonimine hydrochloride). In this study, we investigated the structure-activity relationships of Neoechinulin A and a set of its analogues by using assays to measure anti-nitration and antioxidant activities and cytoprotection against SIN-1-induced PC12 cell death. The presence of the diketopiperazine ring was essential for both the antioxidant and anti-nitration activities of Neoechinulin A derivatives. Nevertheless, a derivative lacking the diketopiperazine ring could still protect PC12 cells against SIN-1 cytotoxicity. An acyclic analogue completely lost the cytoprotective effect while retaining its antioxidant/anti-nitration activities. Pre-incubation of the cells with Neoechinulin A for at least 12 hours was essential for the cells to gain SIN-1 resistance. These results suggest that Neoechinulin A endows the cells with cytoprotection through a biological effect different from the apparent antioxidant/anti-nitration activities.
Neoechinulin A suppresses amyloid-beta oligomer-induced microglia activation and thereby protects PC-12 cells from inflammation-mediated toxicity.[Pubmed:23261590]
Neurotoxicology. 2013 Mar;35:30-40.
A pathological hallmark of Alzheimer's disease (AD), aggregation and deposition of amyloid-beta peptides, has been recognized as a potent activator of microglia-mediated neuroinflammation and neuronal dysfunction. Therefore, downregulation of microglial activation has a significant therapeutic demand. In this study, focus was given to evaluate the ability of Neoechinulin A, an indole alkaloid isolated from marine-derived Microsporum sp., to attenuate microglial activation by oligomeric amyloid-beta 1-42 (Abeta42). Neoechinulin A treatment significantly inhibited the generation of reactive oxygen and nitrogen species in Abeta42-activated BV-2 microglia cells. In addition, we found that Neoechinulin A significantly suppressed the production of neurotoxic inflammatory mediator tumour necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta), interleukin-6 (IL-6), and prostaglandin E2 (PGE2) in activated BV-2 cells. Moreover, the treatment downregulated the protein and gene expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-alpha, IL-1beta and IL-6. Further, activated microglia-mediated apoptosis of PC-12 pheochromocytoma cells was significantly repressed by Neoechinulin A. The molecular mechanism studies suggested that Neoechinulin A may block the phosphorylation of mitogen-activated protein kinase (MAPK) molecule p38, apoptosis signal-regulating kinase 1 (ASK-1) and nuclear translocation of nuclear factor-kappaB (NF-kappaB) p65 and p50 subunits. Regulation of these signalling pathways have most probably contributed to the anti-inflammatory activity of Neoechinulin A. Collectively, these results suggest that with further studies Neoechinulin A have a potential to be developed as a modulator of neuroinflammatory process in AD.


