PMX 205C5a receptor peptide antagonist CAS# 514814-49-4 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 514814-49-4 | SDF | Download SDF |
| PubChem ID | 122173136 | Appearance | Powder |
| Formula | C45H62N10O6 | M.Wt | 839.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
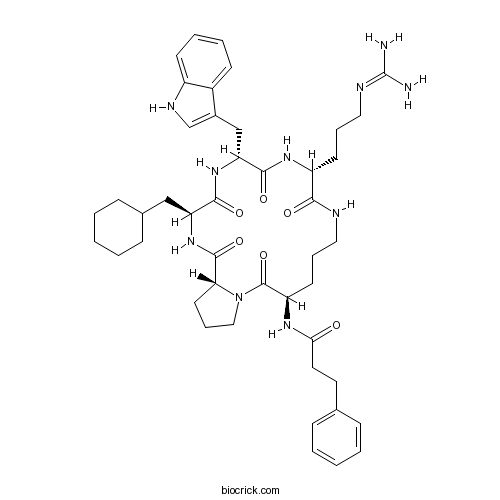
| Sequence | XPXWR (Modifications: X-1 = N2-(1-Oxo-3-phenylpropyl)-Orn, | ||
| Chemical Name | N-[(3R,9R,12R,15S,18R)-15-(cyclohexylmethyl)-9-[3-(diaminomethylideneamino)propyl]-12-(1H-indol-3-ylmethyl)-2,8,11,14,17-pentaoxo-1,7,10,13,16-pentazabicyclo[16.3.0]henicosan-3-yl]-3-phenylpropanamide | ||
| SMILES | C1CCC(CC1)CC2C(=O)NC(C(=O)NC(C(=O)NCCCC(C(=O)N3CCCC3C(=O)N2)NC(=O)CCC4=CC=CC=C4)CCCN=C(N)N)CC5=CNC6=CC=CC=C65 | ||
| Standard InChIKey | VATFHFJULBPYLM-JTDOPDNRSA-N | ||
| Standard InChI | InChI=1S/C45H62N10O6/c46-45(47)49-24-9-18-34-40(57)48-23-10-19-35(51-39(56)22-21-29-12-3-1-4-13-29)44(61)55-25-11-20-38(55)43(60)54-36(26-30-14-5-2-6-15-30)41(58)53-37(42(59)52-34)27-31-28-50-33-17-8-7-16-32(31)33/h1,3-4,7-8,12-13,16-17,28,30,34-38,50H,2,5-6,9-11,14-15,18-27H2,(H,48,57)(H,51,56)(H,52,59)(H,53,58)(H,54,60)(H4,46,47,49)/t34-,35-,36+,37-,38-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent C5a receptor peptide antagonist (IC50 = 31 nM). Ameliorates experimentally-induced colon inflammation in mice. Reduces fibrillar amyloid deposits, decreases hyperphosphorylated tau levels and rescues cognitive function in a mouse model of Alzheimer's Disease. Also improves hindlimb grip strength and slows disease progression in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Orally active and brain penetrant. |

PMX 205 Dilution Calculator

PMX 205 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PMX205 is a cyclic hexapeptide (c[Arg-Trp-D-Cha-Pro-Orn]-Hca), which is an antagonist of the C5a complement receptor. [1]
Human C5a, a complement-derived anaphylatoxin, is a potent mediator of human leukocyte chemotaxis. C5a in particular is a potent inflammatory mediator and signalling through CD88 mediates chemotaxis of leukocytes, vascular permeability could be increased, mast cells release inflammatory mediator and smooth muscle cell contracts and other activities, but all the activities contribute to inflammation and potent tissue damage. C5a-mediated leukocyte chemotaxis release from cytochalasin B-treated cells closely paralleled uptake of the ligand, clearly indicating that it is a receptor-C5a interaction that leads to stimulation of these cellular responses. [2]
PMX205 have been shown to be effective in causing a significant reduction in fibrillar amyloid deposits and activated glia in two mouse models of Alzheimer’s disease. PMX205 -treated rats exhibited reduced striatal lesion size, apoptosis, neutrophil infiltration, and hemorrhage.[3]
PMX205 is used as anti-inflammatory drug. PMX205 significantly prevented DSS-induced colon inflammation, and dealed with PMX205, subjected rats had lower pro-inflammatory. Additionally, the levels of anti-inflammatory cytokines IL-4 and IL-10 were increased. PMX205 did not affect C5a’s levels. The positive effect of PMX205 was seen in two strains of mice of differing sensitivities to DSS inflammation. Pharmacological inhibition of C5a activity by PMX205 is efficacious in preventing DSS-induced colitis.[3]
References:
[1] Delisle Milton RC, Milton SC, Chamberlin AR. Improving the Fmoc Solid Phase Synthesis of the Cyclic Hexapeptide Complement C5a Antagonist, PMX205. Int J Pept Res Ther. 2011 Dec;17(4):337-342.
[2] Chenoweth DE, Hugli TE. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943-7.
[3] Jain U, Woodruff TM, Stadnyk AW. The C5a receptor antagonist PMX205 ameliorates experimentally induced colitis associated with increased IL-4 and IL-10. Br J Pharmacol. 2013 Jan;168(2):488-501.
- Cimetidine
Catalog No.:BCC4527
CAS No.:51481-61-9
- Deoxynivalenol
Catalog No.:BCC7832
CAS No.:51481-10-8
- Cyclo(Tyr-Phe)
Catalog No.:BCN2423
CAS No.:5147-17-1
- COG 133
Catalog No.:BCC1047
CAS No.:514200-66-9
- 3-Methyladenine
Catalog No.:BCC3714
CAS No.:5142-23-4
- Odonicin
Catalog No.:BCN5637
CAS No.:51419-51-3
- Chikusetsusaponin IVa
Catalog No.:BCN3432
CAS No.:51415-02-2
- Alrestatin
Catalog No.:BCC6663
CAS No.:51411-04-2
- Canthaxanthin
Catalog No.:BCC8139
CAS No.:514-78-3
- Biperiden
Catalog No.:BCC4274
CAS No.:514-65-8
- Ferruginol
Catalog No.:BCN3155
CAS No.:514-62-5
- Euphol
Catalog No.:BCN7790
CAS No.:514-47-6
- H-Tyr(tBu)-OMe.HCl
Catalog No.:BCC2672
CAS No.:51482-39-4
- Sclareol
Catalog No.:BCN2395
CAS No.:515-03-7
- (+)-Turicine
Catalog No.:BCC8361
CAS No.:515-24-2
- Cochinchinenin A
Catalog No.:BCN3496
CAS No.:221696-69-1
- Adiantulupanone
Catalog No.:BCN7360
CAS No.:51511-05-8
- GW 803430
Catalog No.:BCC7897
CAS No.:515141-51-2
- Vitexin argininate
Catalog No.:BCC8179
CAS No.:51542-56-4
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Neoechinulin A
Catalog No.:BCN5638
CAS No.:51551-29-2
- DMH4
Catalog No.:BCC6196
CAS No.:515880-75-8
- Cuspidiol
Catalog No.:BCN3942
CAS No.:51593-96-5
- Methylmalonate
Catalog No.:BCC7986
CAS No.:516-05-2
The C5a receptor antagonist PMX205 ameliorates experimentally induced colitis associated with increased IL-4 and IL-10.[Pubmed:22924972]
Br J Pharmacol. 2013 Jan;168(2):488-501.
BACKGROUND AND PURPOSE: Anti-complement therapies have not been advanced for treating the inflammatory bowel diseases (IBDs) despite a growing body of evidence that blocking C5a protects against induced colitis in rodents. The purpose of this study was to further build on this evidence by examining the efficacy, mechanism and specificity of a potent, non-competitive and orally active C5a receptor (CD88) antagonist, PMX205, in the dextran sulphate sodium (DSS) model of murine innate colitis. EXPERIMENTAL APPROACH: Mice with DSS added to their drinking water were orally administered 100 or 200 mug day(-1) PMX205 in prophylactic and therapeutic regimens. Clinical illness, colon histology and local generation of inflammatory mediators were measured to evaluate the impact of PMX205 on disease. KEY RESULTS: PMX205 significantly prevented DSS-induced colon inflammation in both regimens, associated with lower pro-inflammatory cytokine production and nitrotyrosine staining in colon sections. Additionally, the levels of anti-inflammatory cytokines IL-4 and IL-10 were increased. PMX205 had no significant effect on C5a levels. The beneficial effect of PMX205 was seen in two strains of mice of differing sensitivities to DSS inflammation, but was inactive in mice lacking CD88. CONCLUSIONS AND IMPLICATIONS: Pharmacological inhibition of C5a activity by PMX205 is efficacious in preventing DSS-induced colitis, providing further evidence that targeting CD88 in IBD patients could be a valuable therapeutic option.
Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease.[Pubmed:19561098]
J Immunol. 2009 Jul 15;183(2):1375-83.
Alzheimer's disease (AD) is an age-related dementia, characterized by amyloid plaques, neurofibrillary tangles, neuroinflammation, and neuronal loss in the brain. Components of the complement system, known to produce a local inflammatory reaction, are associated with the plaques and tangles in AD brain, and thus a role for complement-mediated inflammation in the acceleration or progression of disease has been proposed. A complement activation product, C5a, is known to recruit and activate microglia and astrocytes in vitro by activation of a G protein-coupled cell-surface C5aR. Here, oral delivery of a cyclic hexapeptide C5a receptor antagonist (PMX205) for 2-3 mo resulted in substantial reduction of pathological markers such as fibrillar amyloid deposits (49-62%) and activated glia (42-68%) in two mouse models of AD. The reduction in pathology was correlated with improvements in a passive avoidance behavioral task in Tg2576 mice. In 3xTg mice, PMX205 also significantly reduced hyperphosphorylated tau (69%). These data provide the first evidence that inhibition of a proinflammatory receptor-mediated function of the complement cascade (i.e., C5aR) can interfere with neuroinflammation and neurodegeneration in AD rodent models, suggesting a novel therapeutic target for reducing pathology and improving cognitive function in human AD patients.
Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity.[Pubmed:15044616]
Mol Pharmacol. 2004 Apr;65(4):868-79.
Human C5a is a plasma protein with potent chemoattractant and pro-inflammatory properties, and its overexpression correlates with severity of inflammatory diseases. C5a binds to its G protein-coupled receptor (C5aR) on polymorphonuclear leukocytes (PMNLs) through a high-affinity helical bundle and a low-affinity C terminus, the latter being solely responsible for receptor activation. Potent and selective C5a antagonists are predicted to be effective anti-inflammatory drugs, but no pharmacophore for small molecule antagonists has yet been developed, and it would significantly aid drug design. We have hypothesized that a turn conformation is important for activity of the C terminus of C5a and herein report small cyclic peptides that are stable turn mimics with potent antagonism at C5aR on human PMNLs. A comparison of solution structures for the C terminus of C5a, small acyclic peptide ligands, and cyclic antagonists supports the importance of a turn for receptor binding. Competition between a cyclic antagonist and either C5a or an acyclic agonist for C5aR on PMNLs supports a common or overlapping binding site on the C5aR. Structure-activity relationships for 60 cyclic analogs were evaluated by competitive radioligand binding with C5a (affinity) and myeloperoxidase release (antagonist potency) from human PMNLs, with 20 compounds having high antagonist potencies (IC(50), 20 nM-1 microM). Computer modeling comparisons reveal that potent antagonists share a common cyclic backbone shape, with affinity-determining side chains of defined volume projecting from the cyclic scaffold. These results define a new pharmacophore for C5a antagonist development and advance our understanding of ligand recognition and receptor activation of this G protein-coupled receptor.


