CanthaxanthinCAS# 514-78-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 514-78-3 | SDF | Download SDF |
| PubChem ID | 5281227 | Appearance | Powder |
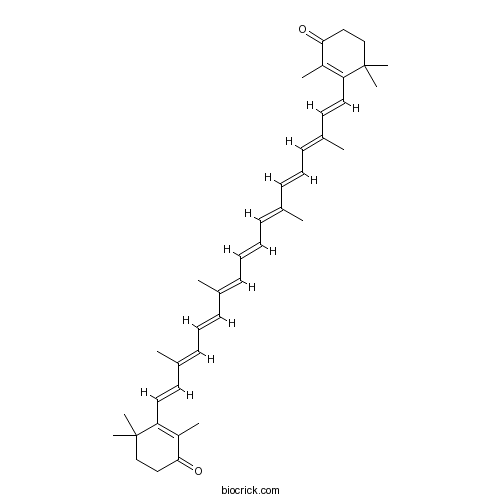
| Formula | C40H52O2 | M.Wt | 564.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | E 161g; all-trans-Canthaxanthin | ||
| Solubility | DMSO : 1 mg/mL (1.77 mM; ultrasonic and warming and heat to 80°C) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2,4,4-trimethyl-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-3-oxocyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohex-2-en-1-one | ||
| SMILES | CC1=C(C(CCC1=O)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC2=C(C(=O)CCC2(C)C)C)C)C | ||
| Standard InChIKey | FDSDTBUPSURDBL-DKLMTRRASA-N | ||
| Standard InChI | InChI=1S/C40H52O2/c1-29(17-13-19-31(3)21-23-35-33(5)37(41)25-27-39(35,7)8)15-11-12-16-30(2)18-14-20-32(4)22-24-36-34(6)38(42)26-28-40(36,9)10/h11-24H,25-28H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,29-15+,30-16+,31-19+,32-20+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Canthaxanthin Dilution Calculator

Canthaxanthin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7705 mL | 8.8527 mL | 17.7054 mL | 35.4108 mL | 44.2635 mL |
| 5 mM | 0.3541 mL | 1.7705 mL | 3.5411 mL | 7.0822 mL | 8.8527 mL |
| 10 mM | 0.1771 mL | 0.8853 mL | 1.7705 mL | 3.5411 mL | 4.4263 mL |
| 50 mM | 0.0354 mL | 0.1771 mL | 0.3541 mL | 0.7082 mL | 0.8853 mL |
| 100 mM | 0.0177 mL | 0.0885 mL | 0.1771 mL | 0.3541 mL | 0.4426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Canthaxanthin is a red-orange carotenoid with various biological activities, such as antioxidant, antitumor properties.
In Vitro:Canthaxanthin enrichment of LDL has the potential to protect cholesterol from oxidation. In addition to its free radical scavenging and antioxidant properties (e.g., the induction of catalase and superoxide dismutase), canthaxanthin shows immunomodulatory activity (e.g. enhancing the proliferation and function of immune competent cells) and plays important role in gap junction communication (e.g. induction of the transmembrane protein connexin)[1]. At concentrations of 0.1 to 1 x 1000 μM, canthaxanthin significantly reduces the overall number of tumor cells. The greatest inhibition is observed at a canthaxanthin concentration of 1000 after 72 h and 96 h of incubation[2].
In Vivo:Canthaxanthin alters the protective ability of tissues against oxidative stress. Canthaxanthin treatment for 15 d at the dose of 14 μg/kg body weight results in hepatic incorporation of the carotenoid, which is maximum in liver and reaches 0.52 ± 0.05 nmol/g liver. Glutathione peroxidase activity is 35% lower and catalase (59%) and manganese superoxide dismutase (28%) activities are higher in canthaxanthin-treated mice than in controls[3]. Canthaxanthin inhibit the growth of mammary tumors in mice and the anti-tumor activity is also influenced by the supplemental dose[4]. Diet supplementation with canthaxanthin for 3 weeks prior to the carcinogen results in a 65% reduction in the number of mammary cancers by a mechanism not involving pro-vitamin A activity[5].
References:
[1]. Esatbeyoglu T, et al. Canthaxanthin: From molecule to function. Mol Nutr Food Res. 2017 Jun;61(6).
[2]. Huang DS, et al. Inhibitory effects of canthaxanthin on in vitro growth of murine tumor cells. Cancer Lett. 1992 Aug 31;65(3):209-13.
[3]. Palozza P, et al. Canthaxanthin supplementation alters antioxidant enzymes and iron concentration in liver of Balb/c mice. J Nutr. 2000 May;130(5):1303-8.
[4]. Chew BP, et al. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999 May-Jun;19(3A):1849-53.
[5]. Grubbs CJ, et al. Effect of canthaxanthin on chemically induced mammary carcinogenesis. Oncology. 1991;48(3):239-45.
- Biperiden
Catalog No.:BCC4274
CAS No.:514-65-8
- Ferruginol
Catalog No.:BCN3155
CAS No.:514-62-5
- Euphol
Catalog No.:BCN7790
CAS No.:514-47-6
- Tirucallol
Catalog No.:BCN7787
CAS No.:514-46-5
- Parkeol
Catalog No.:BCN3728
CAS No.:514-45-4
- Periplogenin
Catalog No.:BCN2656
CAS No.:514-39-6
- Abietic acid
Catalog No.:BCN2728
CAS No.:514-10-3
- Taraxerone
Catalog No.:BCN5636
CAS No.:514-07-8
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Alrestatin
Catalog No.:BCC6663
CAS No.:51411-04-2
- Chikusetsusaponin IVa
Catalog No.:BCN3432
CAS No.:51415-02-2
- Odonicin
Catalog No.:BCN5637
CAS No.:51419-51-3
- 3-Methyladenine
Catalog No.:BCC3714
CAS No.:5142-23-4
- COG 133
Catalog No.:BCC1047
CAS No.:514200-66-9
- Cyclo(Tyr-Phe)
Catalog No.:BCN2423
CAS No.:5147-17-1
- Deoxynivalenol
Catalog No.:BCC7832
CAS No.:51481-10-8
- Cimetidine
Catalog No.:BCC4527
CAS No.:51481-61-9
- PMX 205
Catalog No.:BCC8039
CAS No.:514814-49-4
- H-Tyr(tBu)-OMe.HCl
Catalog No.:BCC2672
CAS No.:51482-39-4
- Sclareol
Catalog No.:BCN2395
CAS No.:515-03-7
- (+)-Turicine
Catalog No.:BCC8361
CAS No.:515-24-2
Retinal toxicity due to canthaxanthin. Case series.[Pubmed:29573837]
Arch Soc Esp Oftalmol. 2018 Aug;93(8):411-415.
INTRODUCTION: Canthaxanthin is a chemical product used to tan the skin. Its most frequent adverse effect is Canthaxanthin retinopathy. PURPOSE/ METHODS: Report, case series. RESULTS: Two female patients, one 42 years-old and the other 72 years-old, with signs of retinopathy due to Canthaxanthin. Complete ophthalmology examinations were carried out. The peripheral fovea birefringent deposits with internal retinal involvement were studied using multimodal imaging. CONCLUSION: Canthaxanthin retinopathy is rare. Multimodal imaging may provide important data for the differential diagnosis of crystalline retinopathy.


