Abietic acidCAS# 514-10-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

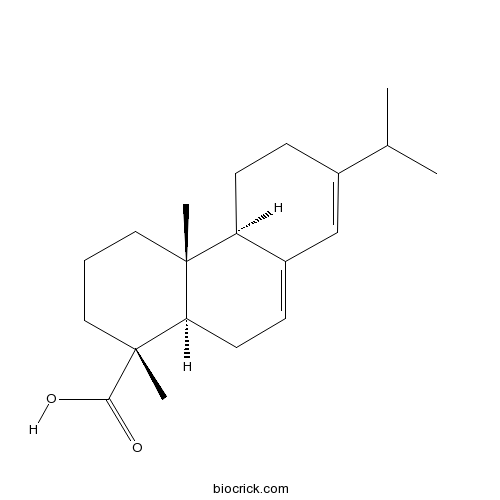
| Cas No. | 514-10-3 | SDF | Download SDF |
| PubChem ID | 10569 | Appearance | Powder |
| Formula | C20H30O2 | M.Wt | 302.45 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4aR,4bR,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylic acid | ||
| SMILES | CC(C)C1=CC2=CCC3C(C2CC1)(CCCC3(C)C(=O)O)C | ||
| Standard InChIKey | RSWGJHLUYNHPMX-ONCXSQPRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Abietic acid, an abietane diterpenoid, inhibited soybean 5-lipoxygenase with an IC50 of 29.5 ± 1.29 μM.Abietic acid acts as a PPARα/γ dual activator to inhibit UVB-induced MMP-1 expression and age-related inflammation by suppressing NF-κB and the MAPK/AP-1 pathway and can be a useful agent for improving skin photoageing. Abietic acid can be used not only for anti-inflammation but also for regulating lipid metabolism and atherosclerosis. |
| Targets | NF-kB | p65 | PPAR | MMP(e.g.TIMP) | MAPK | AP-1 | NO | PGE | COX | IL Receptor |
| In vitro | Tetrahydroabietic Acid, a Reduced Abietic Acid, Inhibits the Production of Inflammatory Mediators in RAW264.7 Macrophages Activated with Lipopolysaccharide.[Pubmed: 20216944 ]J Clin Biochem Nutr. 2010 Mar;46(2):119-25.Abietic acid (AA), the main component of the rosin fraction of oleoresin synthesized by conifer species, has been reported to have anti-inflammatory effects. AA is a weak contact allergen; however, compounds resulting from its oxidation by air elicit stronger allergic response. Hydrogenation of the conjugated double bonds of AA, as in tetrahydroAbietic acid (THAA), decreases its susceptibility to air oxidation and would thus reduce the allergenicity of AA. The aim of this study was to investigate whether THAA could exert anti-inflammatory effects to the same extent as AA in RAW264.7 macrophages activated with the endotoxin lipopolysaccharide (LPS). |
| In vivo | Abietic acid has an anti-obesity effect in mice fed a high-fat diet.[Pubmed: 21812648]J Med Food. 2011 Sep;14(9):1052-6.We investigated the anti-obesity effect of Abietic acid in mice fed a high-fat diet with emphasis on changes in adipogenesis in epididymal adipose tissues. |
| Kinase Assay | Abietic acid inhibits UVB-induced MMP-1 expression in human dermal fibroblast cells through PPARα/γ dual activation.[Pubmed: 25496486]Exp Dermatol. 2015 Feb;24(2):140-5.Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors and consist of three isotypes: PPARα, PPARβ/δ and PPARγ. PPARs are expressed in various cell types in the skin, including keratinocytes, fibroblasts and infiltrating immune cells. Thus, these receptors are highly studied in dermato-endocrine research, and their ligands are targets for the treatment of various skin disorders, such as photoageing and chronological ageing of skin. |
| Cell Research | Abietic acid activates peroxisome proliferator-activated receptor-gamma (PPARgamma) in RAW264.7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism.[Pubmed: 12935909]FEBS Lett. 2003 Aug 28;550(1-3):190-4.Abietic acid is one of the terpenoids, which are multifunctional natural compounds. It has been reported that Abietic acid suppresses effects on inflammation. However, the mechanism underlying the anti-inflammatory effects remains unclear. |

Abietic acid Dilution Calculator

Abietic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3063 mL | 16.5317 mL | 33.0633 mL | 66.1266 mL | 82.6583 mL |
| 5 mM | 0.6613 mL | 3.3063 mL | 6.6127 mL | 13.2253 mL | 16.5317 mL |
| 10 mM | 0.3306 mL | 1.6532 mL | 3.3063 mL | 6.6127 mL | 8.2658 mL |
| 50 mM | 0.0661 mL | 0.3306 mL | 0.6613 mL | 1.3225 mL | 1.6532 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3306 mL | 0.6613 mL | 0.8266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Taraxerone
Catalog No.:BCN5636
CAS No.:514-07-8
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Budesonide
Catalog No.:BCC4767
CAS No.:51333-22-3
- Soyasaponin Bb
Catalog No.:BCN2598
CAS No.:51330-27-9
- Aloe-emodin-glucoside
Catalog No.:BCC8130
CAS No.:29010-56-8
- Tizanidine
Catalog No.:BCC4082
CAS No.:51322-75-9
- 27-Hydroxymangiferonic acid
Catalog No.:BCN4626
CAS No.:5132-66-1
- Alpha-Eudesmol
Catalog No.:BCC8272
CAS No.:473-16-5
- L-NAME hydrochloride
Catalog No.:BCC2865
CAS No.:51298-62-5
- Periplogenin
Catalog No.:BCN2656
CAS No.:514-39-6
- Parkeol
Catalog No.:BCN3728
CAS No.:514-45-4
- Tirucallol
Catalog No.:BCN7787
CAS No.:514-46-5
- Euphol
Catalog No.:BCN7790
CAS No.:514-47-6
- Ferruginol
Catalog No.:BCN3155
CAS No.:514-62-5
- Biperiden
Catalog No.:BCC4274
CAS No.:514-65-8
- Canthaxanthin
Catalog No.:BCC8139
CAS No.:514-78-3
- Alrestatin
Catalog No.:BCC6663
CAS No.:51411-04-2
- Chikusetsusaponin IVa
Catalog No.:BCN3432
CAS No.:51415-02-2
- Odonicin
Catalog No.:BCN5637
CAS No.:51419-51-3
- 3-Methyladenine
Catalog No.:BCC3714
CAS No.:5142-23-4
- COG 133
Catalog No.:BCC1047
CAS No.:514200-66-9
Abietic acid activates peroxisome proliferator-activated receptor-gamma (PPARgamma) in RAW264.7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism.[Pubmed:12935909]
FEBS Lett. 2003 Aug 28;550(1-3):190-4.
Abietic acid is one of the terpenoids, which are multifunctional natural compounds. It has been reported that Abietic acid suppresses effects on inflammation. However, the mechanism underlying the anti-inflammatory effects remains unclear. The present work indicates that Abietic acid suppresses the protein expression of tumor necrosis factor-alpha and cyclooxygenase 2, which are involved in inflammation, in lipopolysaccharide-stimulated macrophages. Moreover, this effect resembles that of thiazolidinedione, a synthetic peroxisome proliferator-activated receptor-gamma (PPARgamma) ligand. Indeed, Abietic acid activates PPARgamma in luciferase reporter assays. The activity of Abietic acid induces PPARgamma target gene expression in RAW264.7 macrophages and 3T3-L1 adipocytes. These data indicate that Abietic acid is a PPARgamma ligand and that its anti-inflammatory effect is partly due to the activation of PPARgamma in stimulated macrophages. The present work suggests a novel possibility that Abietic acid, a naturally occurring compound, can be used not only for anti-inflammation but also for regulating lipid metabolism and atherosclerosis.
Abietic acid has an anti-obesity effect in mice fed a high-fat diet.[Pubmed:21812648]
J Med Food. 2011 Sep;14(9):1052-6.
We investigated the anti-obesity effect of Abietic acid in mice fed a high-fat diet with emphasis on changes in adipogenesis in epididymal adipose tissues. Male C57BL/6J mice were divided into four groups and fed a normal diet, a high-fat diet (HFD), or HFD plus oral administration of Abietic acid (20 mg/kg of body weight/day [LA] or 40 mg/kg of body weight/day [HA]) for 8 weeks. Compared with the HFD group, mice orally administered 40 mg of Abietic acid/kg of body weight/day exhibited significantly decreased body weight and adipose tissue weights. Serum triglyceride concentrations in the HA group were significantly lower than those in the HFD group, as were the levels of serum insulin and leptin. Hematoxylin and eosin staining revealed that epididymal adipose tissue mass was decreased by Abietic acid administration. Abietic acid also inhibited the protein expression of sterol regulatory element-binding protein-1c, CCAAT/enhancer-binding protein alpha, and CD36 in epididymal adipose tissues, which are up-regulated by HFDs. These data demonstrate that Abietic acid has an anti-obesity effect in mice mediated by the regulation of adipogenesis.
Abietic acid inhibits UVB-induced MMP-1 expression in human dermal fibroblast cells through PPARalpha/gamma dual activation.[Pubmed:25496486]
Exp Dermatol. 2015 Feb;24(2):140-5.
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors and consist of three isotypes: PPARalpha, PPARbeta/delta and PPARgamma. PPARs are expressed in various cell types in the skin, including keratinocytes, fibroblasts and infiltrating immune cells. Thus, these receptors are highly studied in dermato-endocrine research, and their ligands are targets for the treatment of various skin disorders, such as photoageing and chronological ageing of skin. Intensive studies have revealed that PPARalpha/gamma functions in photoageing and age-related inflammation by regulating matrix metalloproteinases (MMPs) via nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1). However, the detailed mechanism of PPARalpha/gamma's role in photoageing has not yet been elucidated. In this study, we confirmed that Abietic acid (AA) is a PPARalpha/gamma dual ligand and significantly decreased UVB-induced MMP-1 expression by downregulating UVB-induced MAPK signalling and downstream transcription factors, subsequently reducing IkappaBalpha degradation and blocking NF-kappaB p65 nuclear translocation in Hs68 human dermal fibroblast cells. Treatment of cells with AA and GW6471 or bisphenol A diglycidyl ether (BADGE), PPARalpha or PPARgamma antagonists, respectively, reversed the effect on UVB-induced MMP-1 expression and inflammatory signalling pathway activation. Taken together, our data suggest that AA acts as a PPARalpha/gamma dual activator to inhibit UVB-induced MMP-1 expression and age-related inflammation by suppressing NF-kappaB and the MAPK/AP-1 pathway and can be a useful agent for improving skin photoageing.
Tetrahydroabietic Acid, a Reduced Abietic Acid, Inhibits the Production of Inflammatory Mediators in RAW264.7 Macrophages Activated with Lipopolysaccharide.[Pubmed:20216944]
J Clin Biochem Nutr. 2010 Mar;46(2):119-25.
Abietic acid (AA), the main component of the rosin fraction of oleoresin synthesized by conifer species, has been reported to have anti-inflammatory effects. AA is a weak contact allergen; however, compounds resulting from its oxidation by air elicit stronger allergic response. Hydrogenation of the conjugated double bonds of AA, as in tetrahydroAbietic acid (THAA), decreases its susceptibility to air oxidation and would thus reduce the allergenicity of AA. The aim of this study was to investigate whether THAA could exert anti-inflammatory effects to the same extent as AA in RAW264.7 macrophages activated with the endotoxin lipopolysaccharide (LPS). THAA and AA inhibited the production of nitric oxide (NO) and prostaglandin E(2) by suppressing the expression of inducible NO synthase and cyclooxygenase-2, respectively, in LPS-activated RAW264.7 macrophages. They also inhibited the LPS-induced production of interleukin (IL)-1beta, IL-6, and tumor necrosis factor-alpha. Both THAA and AA prevented the LPS-induced nuclear translocation of the nuclear factor-kappaB/p65 subunit, suggesting that THAA may inhibit the production of pro-inflammatory mediators through the same mechanism as AA. In comparison, the anti-inflammatory effects of THAA and AA were almost identical, indicating that THAA retains the anti-inflammatory activity of AA at least in LPS-activated RAW264.7 macrophages.


