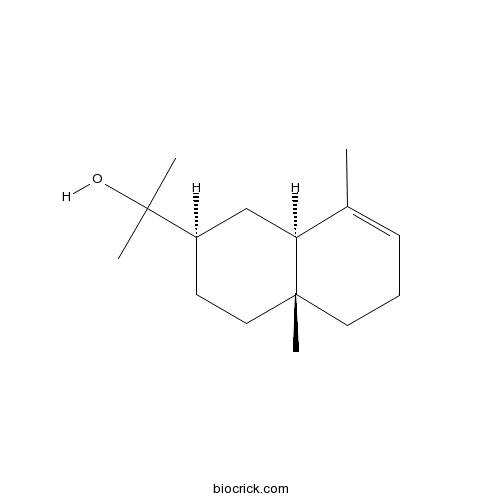
Alpha-EudesmolCAS# 473-16-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 473-16-5 | SDF | Download SDF |
| PubChem ID | 92762 | Appearance | Powder |
| Formula | C15H26O | M.Wt | 222.37 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | α-Eudesmol;a-Eudesmol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(2R,4aR,8aR)-4a,8-dimethyl-2,3,4,5,6,8a-hexahydro-1H-naphthalen-2-yl]propan-2-ol | ||
| SMILES | CC1=CCCC2(C1CC(CC2)C(C)(C)O)C | ||
| Standard InChIKey | FCSRUSQUAVXUKK-VNHYZAJKSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-11-6-5-8-15(4)9-7-12(10-13(11)15)14(2,3)16/h6,12-13,16H,5,7-10H2,1-4H3/t12-,13+,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alpha-Eudesmol Dilution Calculator

Alpha-Eudesmol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.497 mL | 22.485 mL | 44.9701 mL | 89.9402 mL | 112.4252 mL |
| 5 mM | 0.8994 mL | 4.497 mL | 8.994 mL | 17.988 mL | 22.485 mL |
| 10 mM | 0.4497 mL | 2.2485 mL | 4.497 mL | 8.994 mL | 11.2425 mL |
| 50 mM | 0.0899 mL | 0.4497 mL | 0.8994 mL | 1.7988 mL | 2.2485 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4497 mL | 0.8994 mL | 1.1243 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-NAME hydrochloride
Catalog No.:BCC2865
CAS No.:51298-62-5
- 7,4'-Di-O-methylapigenin
Catalog No.:BCN5634
CAS No.:5128-44-9
- 5,7-Diacetoxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1432
CAS No.:5128-43-8
- Afzelechin 3-O-xyloside
Catalog No.:BCN7774
CAS No.:512781-45-2
- 2,6-Dimethyl-3,7-octadiene-2,6-diol
Catalog No.:BCN5633
CAS No.:51276-34-7
- Gallocatechin gallate
Catalog No.:BCN6803
CAS No.:5127-64-0
- Amsacrine
Catalog No.:BCC4309
CAS No.:51264-14-3
- N-Phthaloyl-Phe-OH
Catalog No.:BCC3016
CAS No.:5123-55-7
- Wighteone
Catalog No.:BCN5632
CAS No.:51225-30-0
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- 27-Hydroxymangiferonic acid
Catalog No.:BCN4626
CAS No.:5132-66-1
- Tizanidine
Catalog No.:BCC4082
CAS No.:51322-75-9
- Aloe-emodin-glucoside
Catalog No.:BCC8130
CAS No.:29010-56-8
- Soyasaponin Bb
Catalog No.:BCN2598
CAS No.:51330-27-9
- Budesonide
Catalog No.:BCC4767
CAS No.:51333-22-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
- Taraxerone
Catalog No.:BCN5636
CAS No.:514-07-8
- Abietic acid
Catalog No.:BCN2728
CAS No.:514-10-3
- Periplogenin
Catalog No.:BCN2656
CAS No.:514-39-6
Discovery of three novel sesquiterpene synthases from Streptomyces chartreusis NRRL 3882 and crystal structure of an alpha-eudesmol synthase.[Pubmed:30928538]
J Biotechnol. 2019 Mar 27;297:71-77.
With more than 50,000 members, terpenoids are one of the most important classes of natural products and show an enormous diversity. Due to their unique odors and specific bioactivities they already find wide application in the flavor, fragrance and pharma industries. Since most terpenoids can only be obtained by natural product extraction, the discovery of biosynthetic genes for the generation of terpene diversity becomes increasingly important. This study describes the discovery of three novel sesquiterpene synthases from Streptomyces chartreusis with preference for the formation of germacradiene-11-ol, Alpha-Eudesmol and alpha-amorphene respectively. The Alpha-Eudesmol synthase showed formation of 10-epi-delta-eudesmol and elemol as side products. Eudesmol-isomers are known to have repellent activity, which makes this enzyme a potential catalyst for products for the prevention of mosquito-related disease. The determination of the structure of the apo-enzyme of Alpha-Eudesmol synthase from S. chartreusis provides the first structural insights into an eudesmol-forming enzyme.
Essential oils and ethanol extract from Camellia nitidissima and evaluation of their biological activity.[Pubmed:30483003]
J Food Sci Technol. 2018 Dec;55(12):5075-5081.
Camellia nitidissima, a well-known species of yellow Camellia, has undergone commercial cultivation as a new tea resource recently. Herein, the composition, antioxidant and antimicrobial activities of the essential oil and ethanol extract of C. nitidissima were investigated. The essential oils from the leaves and flowers of C. nitidissima were obtained by hydro-distillation. A total of 56 and 34 constituents accounting for 77.5 and 96.8% of the oils were identified by GC-MS. Linalool (35.8%), phytol (7.9%), cis-geranyl acetone (7.3%) and methyl salicylate (6.8%) were found to be the primary components in the leaf oil, while the flower oil was rich in Alpha-Eudesmol (34.3%), gamma-eudesmol (31.5%) and linalool (11.1%). The ethanol extract of C. nitidissima leaves contained 281.04 mg gallic acid equivalent/g of total phenols. The antioxidant activities of the two oils and extract were evaluated by DPPH and ABTS radical-scavenging assays. The IC50 values varied from 17.4 (extract) to 720.3 mug/mL (flower oil) for DPPH and from 28.8(extract) to 889.6 mug/mL (flower oil) for ABTS. Both essential oils exhibited moderate antioxidant activities, and the extract possessed strong effects close to ascorbic acid. Additionally, the antimicrobial activities of the oils and extract against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa were evaluated by agar dilution assay. No considerable bactericidal activities were observed for either essential oil or extract compared with ampicillin and tobramycin standards. The results indicated the extract was more efficient than the two essential oils against S. aureus (MIC = 0.625 mg/mL) and B. subtilis (MIC = 1.25 mg/mL).
Biological activity of Cymbopogon schoenanthus essential oil.[Pubmed:30294213]
Saudi J Biol Sci. 2017 Nov;24(7):1458-1464.
Introduction: A number of plant species, including Cymbopogon schoenanthus, are traditionally used for the treatment of various diseases. C. schoenanthus is currently, traded in the Saudi markets, and thought to have medicinal value. This study aimed at investigating the biological activities of C. schoenanthus against both Gram-positive and Gram-negative bacteria and to identify its chemical ingredients. Materials and methods: The inhibitory effects of water extracts of C. schoenanthus essential oils were evaluated against ten isolates of both Gram-positive and Gram-negative bacteria using the agar well diffusion and dilution methods. The minimum inhibitory concentration (MIC) was assayed using the Broth microdilution test on five of the ten isolates. The death rates were determined by the time kill assay, done according to the Clinical Laboratory Standards Institute (CLSI) guidelines. The chemical composition of the essential oils of the plant was performed using GC/MS. Results: The C. schoenanthus essential oil was effective against Escherichia coli, Staphylococcus aureus, methicillin-sensitive (MSSA) S. aureus (MRSA) and Klebsiella pneumoniae. The essential oil was not effective against Staphylococcus saprophyticus at the highest concentration applied of >150 mug/ml. The MIC values were as follows: 9.37 mug/ml for E. coli 4.69 mug/ml for S. aureus (MRSA), 2.34 mg/ml for MSSA and 2.34 mug/ml for K. pneumoniae. The time-kill assay indicated that there was a sharp time dependent decline in K. pneumoniae counts in the presence of the oil. This is in contrast to a gradual decline in the case of S. aureus under the same conditions. The eight major components of the essential oil were: piperitone (14.6%), cyclohexanemethanol (11.6%), beta-elemene (11.6%), Alpha-Eudesmol (11.5%), elemol (10.8%), beta-eudesmol (8.5%), 2-naphthalenemethanol (7.1%) and gamma-eudesmol (4.2%). Conclusion: The results of the present study provide a scientific validation for the traditional use of C. schoenanthus as an antibacterial agent. Future work is needed to investigate and explore its application in the environmental and medical fields. In addition, to evaluating the efficacy of the individual ingredients separately to better understand the underlying mechanism.
Development of supercritical CO2 extraction of bioactive phytochemicals from black poplar (Populus nigra L.) buds followed by GC-MS and UHPLC-DAD-QqTOF-MS.[Pubmed:29852355]
J Pharm Biomed Anal. 2018 Sep 5;158:15-27.
The supercritical CO2 (SC-CO2) extraction process of black poplar (Populus nigra L.) buds was optimized (pressure, temperature) based on the yields of major phytochemicals (volatiles and non-volatiles). The optimal settings were 30MPa/60 degrees C. Major volatiles determined by GC-MS in the optimized SC-CO2 extract (mg of benzyl salicylate equivalent (BSE) per 100g of buds) were: pinostrobin chalcone (1574.2), beta-eudesmol (640.8), Alpha-Eudesmol (581.9), 2-methyl-2-butenyl-p-coumarate (289.9), pentyl-p-coumarate (457.0), gamma-eudesmol (294.4), and benzyl salicylate (289.2). Partial qualitative similarity was observed between SC-CO2 extracts and corresponding hydrodistilled essential oil dominated by sesquiterpenes, but with lower yields. Major compounds (mg per 100g of buds) identified by UHPLC-DAD-QqTOF-MS in the optimized SC-CO2 extract were: pinostrobin (751.7), pinocembrin (485.6), 3-O-pinobanksin acetate and methyl-butenyl-p-coumarate (290.2; 144.9 of pinobanksin and p-coumaric acid equivalents, respectively). SC-CO2 extraction was found useful for green, efficient and simultaneous extraction of both volatile/non-volatile, bioactive phytochemicals of poplar buds - precursors of poplar-type propolis.
Chemical investigations of male and female leaf extracts from Schinus molle L.[Pubmed:29842798]
Nat Prod Res. 2018 May 29:1-4.
The pepper-tree Schinus molle is an evergreen ornamental plant with various and diversified list of medical uses. In this article we analysed the chemical composition of male and female leaves of this plant during the off-flowering and flowering seasons. The leaf extracts were obtained by using a sequential extraction with solvents of different polarities and the chemical composition was investigated by GC-MS. The results showed a total of twenty-three components, in which elemol is the most abundant constituent followed by bicyclogermacrene, gamma-eudesmol, Alpha-Eudesmol, beta-eudesmol and isocalamendiol. The petroleum ether and diethyl ether extracts from male and female flowering and off-flowering leaves consisted of sesquiterpene hydrocarbons as a major constituent followed by monoterpene hydrocarbons, while the acetone extracts showed a different composition. The obtained results show differences in the chemical composition between male and female and flowering and not flowering.
Incubation of Aquilaria subintegra with Microbial Culture Supernatants Enhances Production of Volatile Compounds and Improves Quality of Agarwood Oil.[Pubmed:29651179]
Indian J Microbiol. 2018 Jun;58(2):201-207.
Incubation with microbial culture supernatants improved essential oil yield from Aquilaria subintegra woodchips. The harvested woodchips were incubated with de man, rogosa and sharpe (MRS) agar, yeast mold (YM) agar medium and six different microbial culture supernatants obtained from Lactobacillus bulgaricus, L. acidophilus, Streptococcus thermophilus, Lactococcus lactis, Saccharomyces carlsbergensis and S. cerevisiae prior to hydrodistillation. Incubation with lactic acid bacteria supernatants provided higher yield of agarwood oil (0.45% w/w) than that obtained from yeast (0.25% w/w), agar media (0.23% w/w) and water (0.22% w/w). The composition of agarwood oil from all media and microbial supernatant incubations was investigated by using gas chromatography-mass spectrometry. Overall, three major volatile profiles were obtained, which corresponded to water soaking (control), as well as, both YM and MRS media, lactic acid bacteria, and yeast supernatant incubations. Sesquiterpenes and their oxygenated derivatives were key components of agarwood oil. Fifty-two volatile components were tentatively identified in all samples. Beta-agarofuran, Alpha-Eudesmol, karanone, alpha-agarofuran and agarospirol were major components present in most of the incubated samples, while S. cerevisiae-incubated A. subintegra provided higher amount of phenyl acetaldehyde. Microbial culture supernatant incubation numerically provided the highest yield of agarwood oil compared to water soaking traditional method, possibly resulting from activity of extracellular enzymes produced by the microbes. Incubation of agarwood with lactic acid bacteria supernatant significantly enhanced oil yields without changing volatile profile/composition of agarwood essential oil, thus this is a promising method for future use.
Chemical composition of essential oil from plants of abandoned mining site of Elba island.[Pubmed:29417841]
Nat Prod Res. 2019 Jan;33(1):143-147.
The essential oil composition of three spontaneous species growing in an abandoned mining of Elba island was analyzed by GC-MS. A total of 194 compounds were identified representing 73.7-100% of the whole oil composition. The essential oils of Cistus salvifolius and Dittrichia viscosa from this site showed different profiles in comparison with those from not polluted area, where oxygenated sesquiterpenes were the main class. Volatiles from D. viscosa growing in ex-mining area presented 10-epi-gamma-eudesmol and Alpha-Eudesmol as main compounds while beta-caryophyllene and limonene were the main ones in not polluted area. Ambroxide and ambrial were the most important compounds in the essential oil from C. salvifolius harvested in polluted area while nonanal and tridecanal were the main compounds in control samples. Oxygenated monoterpenes were the most abundant class from both Lavandula stoechas samples, with fenchone and camphor as main compounds.
Chemical Composition and Bioactivity of Essential Oil from Blepharocalyx salicifolius.[Pubmed:29300307]
Int J Mol Sci. 2018 Jan 4;19(1). pii: ijms19010033.
Natural products represent a source of biologically active molecules that have an important role in drug discovery. The aromatic plant Blepharocalyx salicifolius has a diverse chemical constitution but the biological activities of its essential oils have not been thoroughly investigated. The aims of this paper were to evaluate in vitro cytotoxic, antifungal and antibacterial activities of an essential oil from leaves of B. salicifolius and to identify its main chemical constituents. The essential oil was extracted by steam distillation, chemical composition was determined by gas chromatography/mass spectrometry, and biological activities were performed by a microdilution broth method. The yield of essential oil was 0.86% (w/w), and the main constituents identified were bicyclogermacrene (17.50%), globulol (14.13%), viridiflorol (8.83%), gamma-eudesmol (7.89%) and Alpha-Eudesmol (6.88%). The essential oil was cytotoxic against the MDA-MB-231 (46.60 mug.mL(-1)) breast cancer cell line, being more selective for this cell type compared to the normal breast cell line MCF-10A (314.44 mug.mL(-1)). Flow cytometry and cytotoxicity results showed that this oil does not act by inducing cell death, but rather by impairment of cellular metabolism specifically of the cancer cells. Furthermore, it presented antifungal activity against Paracoccidioides brasiliensis (156.25 mug.mL(-1)) but was inactive against other fungi and bacteria. Essential oil from B. salicifolius showed promising biological activities and is therefore a source of molecules to be exploited in medicine or by the pharmaceutical industry.
Allelopathic Activity and Chemical Composition of Rhynchosia minima (L.) DC. Essential Oil from Egypt.[Pubmed:29064622]
Chem Biodivers. 2018 Jan;15(1).
Aromatic plants attract the attention of many researchers worldwide due to their worthy applications in agriculture, human prosperity, and the environment. Essential oil (EO) could be exploited as effective alternatives to synthetic compounds as it has several biological activities including allelopathy. The EO from the aerial parts of Rhynchosia minima was extracted by hydrodistillation and investigated by gas chromatography/mass spectrometry (GC/MS). Different concentrations (50, 100, 150 and 200 muL L(-1) ) of the EO were prepared for investigation of their allelopathic potential on two weeds; Dactyloctenium aegyptium and Rumex dentatus. Twenty-eight compounds, mainly sesquiterpenes (69.13%) were determined. The major compounds are Alpha-Eudesmol, 2-allyl-5-tert-butylhydroquinone, caryophyllene oxide, trans-caryophyllene, and tau-cadinol. The EO from the R. minima showed a significant inhibition of D. aegyptium and R. dentatus germination, while the seedling growth was stimulated. Therefore, it is not recommended to treat these noxious weeds with the EO of R. minima before the germination. In contrast, the apparent stimulatory effect on the seedling growth offers further studies to use the EO of R. minima to enhance the fitness of different economic crops. However, characterization of green bio-herbicides such as EO (allelochemicals) from wild plants raises a new opportunity for the incorporation of new technology of bio-control against the noxious weeds.
Chemical composition and in vitro antileishmanial and cytotoxic activities of the essential oils of Ocotea dispersa (Nees) Mez and Ocotea odorifera (Vell) Rohwer (Lauraceae).[Pubmed:29022353]
Nat Prod Res. 2017 Oct 12:1-4.
We investigate the chemical composition and the in vitro antileishmanial and cytotoxic activities of the essential oils extracted from the leaves of Ocotea dispersa (Nees) Mez (OD-EO) and Ocotea odorifera (Vell) Rohwer (OO-EO). On the basis of GC-FID and GC-MS, Alpha-Eudesmol (20.9%), valencene (10.2%), delta-elemene (9.3%) and isospathulenol (7.3%) are the major constituents of OD-EO, whereas safrole (36.3%), gamma-cadinene (6.6%), camphor (6.5%) and alpha-copaene (6.0%) are the main constituents of OO-EO. Both OD-EO and OO-EO display significant activity against the promastigote forms of Leishmania amazonensis, with IC50 values of 4.67 +/- 0.95 and 11.67 +/- 2.16 mug/mL, respectively. The 50% cytotoxic concentration (CC50) of OD-EO and OO-EO to mouse peritoneal macrophages is 26.77 +/- 4.06 and 49.52 +/- 1.04 mug/mL, respectively. This is the first report on the chemical composition of the essential oil extracted from the leaves of O. dispersa. Our results suggest that OD-EO and OO-EO are a promising source of new antileishmanial agents.
Transcriptome and metabolome analysis of Ferula gummosa Boiss. to reveal major biosynthetic pathways of galbanum compounds.[Pubmed:28687892]
Funct Integr Genomics. 2017 Nov;17(6):725-737.
Ferula gummosa Boiss. is an industrial and pharmaceutical plant that has been highly recognized for its valuable oleo-gum-resin, namely galbanum. Despite the fabulous value of galbanum, very little information on the genetic and biochemical mechanisms of its production existed. In the present study, the oleo-gum-resin and four organs (root, flower, stem, and leaf) of F. gummosa were assessed in terms of metabolic compositions and the expression of genes involved in their biosynthetic pathways. Results showed that the most accumulation of resin and essential oils were occurred in the roots (13.99 mg/g) and flowers (6.01 mg/g), respectively. While the most dominant compound of the resin was beta-amyrin from triterpenes, the most abundant compounds of the essential oils were alpha-pinene and beta-pinene from monoterpenes and Alpha-Eudesmol and germacrene-D from sesquiterpenes. Transcriptome analysis was performed by RNA sequencing (RNA-seq) for the plant roots and flowers. Differential gene expression analysis showed that 1172 unigenes were differential between two organs that 934 (79.6%) of them were up-regulated in the flowers and 238 (20.4%) unigenes were up-regulated in the roots (FDR
Terpene constituents of the aerial parts, phenolic content, antibacterial potential, free radical scavenging and antioxidant activity of Callistemon citrinus (Curtis) Skeels (Myrtaceae) from Eastern Cape Province of South Africa.[Pubmed:28583128]
BMC Complement Altern Med. 2017 Jun 5;17(1):292.
BACKGROUND: Volatile oil from aromatic plants has been used by ancient Egyptians in embalming for the inhibition of bacterial growth and prevention of decay, Callistemon citrinus is used in traditional therapies for the treatment of bronchitis, cough, inflammation and as an antimicrobial herbs. This study examines the essential constituents of the volatile oils obtained from the aerial parts of the plant as well as its antioxidant activity, free radical scavenging, phenolic content and the antibacterial potential of the oils. METHODS: A portion of 500 g, 250 g and 150 g of the leaves, flowers and stems of this plant respectively were subjected to hydro-distillation process for three hours. The oils collected from the various plant parts were immediately subjected to GC-MS analysis. The overall phenolic content of the leaves oil, radical scavenging, antibacterial action and antioxidant activities of the essential oils of both the leaves and flowers of Callistemon citrinus were determined using standard methods, with free radical DPPH and ABTS as a reference antioxidant. RESULTS: Analyses of the three oils revealed a total of twenty-six components for the leaves oil representing 96.84% of the total oil composition, forty-one components for the flowers oil accounting for 98.92% of the whole composition and ten components for the stem oil amounting to 99.98% of the entire oil constituents. The dominant compounds in the leaves oil were eucalyptol (48.98%) and alpha-terpineol (8.01%), while Alpha-Eudesmol (12.93%), caryophyllene (11.89%), (-)-bornyl-acetate (10.02%) and eucalyptol (8.11%) were the main constituents of the flowers oil. In the same vein, the leading constituents in the stems oil were eucalyptol (56.00%) and alpha-pinene (31.03%). The antioxidant capacities of both the leaves and flowers oils of the plant were evaluated and their IC50 were (1.49 and 1.13) for DPPH and (0.14 and 0.03) for ABTS assay respectively. The antibacterial activities of the oils from the (leaves and flowers) were also examined and were found to have wide range of activities against the bacterial strains used in this study. CONCLUSION: Observations drawn from this experiment shows clearly that the leaves and flowers of Callistemon citrinus possess phenolic compounds and cyclic ether of several pharmacological behaviors.
Integrated Analysis by GC(RI), GC-MS and 1C NMR of Fortunella japonica Leaf Volatiles Obtained by Hydrodistillation, Microwave- assisted Hydrodistillation and Hydrolate Extraction.[Pubmed:30549903]
Nat Prod Commun. 2017 Mar;12(3):431-434.
The chemical composition of the essential oil (EO), microwave extract (ME) and hydrolate extract (HE) from the same batch of leaves of Fortunella japonica, was investigated: by combination of chromatographic (GC, CC) and spectroscopic techniques (GC-MS, 13C NMR). F. japonica essential oil and extracts are complex mixtures of 28-60 compounds being mainly oxygenated sesquiterpenes. The EO composition was dominated by germacrene D (14.9%), beta-elemol (9.1%), cis-guai-6-en-10beta-ol (6.3%), beta-eudesmol (5.5%), and delta-elemene (5.2%). Limonene was the unique monoterpene identified at appreciable amount (7.1%). The extract obtained by microwave assisted hydrodistillation contained as main components: beta-elemol (12.4%), germacrene D (9.9%), cis-guai-6-en- 10beta-ol (9.0%), beta-eudesmol (8.2%), germacra-l(l0),5-dien-4alpha-ol (7.1%) and Alpha-Eudesmol (6.4%). Finally, the highest content of oxygenated sesquiterpenes (near 92%) was found in the hydrolate extract displaying cryptomeridiol (23.3%, but totally absent in the EO and ME), beta-eudesmol (20.6%) and Alpha-Eudesmol (10.7%). Combined analysis by chromatographic and spectroscopic techniques appeared useful for identification of various sesquiterpenols bearing a tertiary alcohol function.
Barks Essential Oil, Secondary Metabolites and Biological Activities of Four Organs of Tunisian Calligonum azel Maire.[Pubmed:27450433]
Chem Biodivers. 2016 Nov;13(11):1527-1536.
This study is the first to investigate the chemical composition of barks essential oil (EO), secondary metabolites and biological activities of the MeOH and infusions extracts of seeds, leaves, barks and roots of Calligonum azel Maire (Polygonaceae) harvested from Tunisian desert. The gas chromatography/mass spectrometry (GC/MS) results showed the presence of fifty-four compounds in barks EO. The major components were: viridiflorol (14.6%), Alpha-Eudesmol (8.65%), trans-caryophyllene (6.72%), elemol (6.63%), beta-eudesmol (6.21%). The obtained results showed that C. azel is a very rich plant in secondary metabolites. High contents in polyphenols, flavonoids and tannins were observed in both extracts of all studied organs. Significant differences were found between both extracts of the four organs. Thus, polyphenols and tannins were more abundant in leaves infusion extract, while, flavonoids showed a high level in barks extract. The antioxidant activity data demonstrated that all extracts showed strong antioxidant and radical scavenging activities. The MeOH extracts presented potential for antibacterial and antifungal activities against all tested microorganisms. The inhibition zones diameters and minimal inhibitrice concentration values were in the range of 9 - 15 mm and 2.5 - 20 mug/ml, respectively. This study demonstrated that C. azel can be regarded as an excellent plant source for natural antimicrobial agents.
Anti-inflammatory activity of the essential oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy from Eastern Cape, South Africa.[Pubmed:27261849]
Asian Pac J Trop Med. 2016 May;9(5):426-31.
OBJECTIVE: To evaluate the essential oil composition and the anti-inflammatory activity of Cymbopogon validus (C. validus) leaves and flowers. METHODS: A total of 300 g of fresh or dry (leaves and flowers) of C. validus were cut into small pieces and subjected to hydro-distillation method for approximately 5 h using the Clevenger apparatus. The extracted essential oils were then used for testing the anti-inflammatory activity. The anti-inflammatory activity was evaluated by using egg albumin-induced paw edema. RESULTS: The extracted oils had the following yields 2.2% for fresh leaves, 2.0% for dry leaves and 2.4% v/w for dry flowers. GC-MS results revealed that the oils contained artemisia ketone (37.5%), linalool (3.2%-29.6%), northujane (4.4%-16.8%), verbenone (13.5%), naphthalene (1.7%-9.6%), delta-cadinene (0.5%-8.1%), hedycaryol (5.4%-7.6%) and Alpha-Eudesmol (6.5%-6.7%) as the major constituents. C. validus essential oils showed significant (P < 0.05) anti-inflammatory effects from the first 30 min after albumin injection compared to aspirin which had a later onset of effect. CONCLUSIONS: The findings of this study show that the essential oil extracted from C. validus fresh or dry leaves and flowers have anti-inflammatory properties; that might be associated with the major components and the minor components found in the essential oils.


