L-NAME hydrochlorideNO synthase inhibitor CAS# 51298-62-5 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

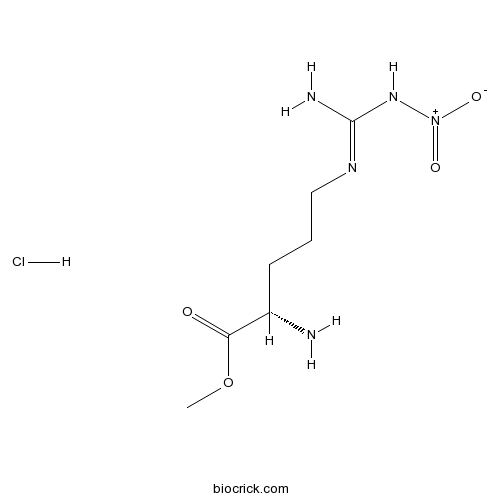
| Cas No. | 51298-62-5 | SDF | Download SDF |
| PubChem ID | 135193 | Appearance | Powder |
| Formula | C7H16ClN5O4 | M.Wt | 269.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NG-Nitroarginine methyl ester hydrochloride | ||
| Solubility | H2O : 125 mg/mL (463.50 mM; Need ultrasonic) DMSO : 100 mg/mL (370.80 mM; Need ultrasonic) H2O : ≥ 32 mg/mL (118.65 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | methyl (2S)-2-amino-5-[[amino(nitramido)methylidene]amino]pentanoate;hydrochloride | ||
| SMILES | COC(=O)C(CCCN=C(N)N[N+](=O)[O-])N.Cl | ||
| Standard InChIKey | QBNXAGZYLSRPJK-JEDNCBNOSA-N | ||
| Standard InChI | InChI=1S/C7H15N5O4.ClH/c1-16-6(13)5(8)3-2-4-10-7(9)11-12(14)15;/h5H,2-4,8H2,1H3,(H3,9,10,11);1H/t5-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NO synthase inhibitor. |

L-NAME hydrochloride Dilution Calculator

L-NAME hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7078 mL | 18.5391 mL | 37.0782 mL | 74.1565 mL | 92.6956 mL |
| 5 mM | 0.7416 mL | 3.7078 mL | 7.4156 mL | 14.8313 mL | 18.5391 mL |
| 10 mM | 0.3708 mL | 1.8539 mL | 3.7078 mL | 7.4156 mL | 9.2696 mL |
| 50 mM | 0.0742 mL | 0.3708 mL | 0.7416 mL | 1.4831 mL | 1.8539 mL |
| 100 mM | 0.0371 mL | 0.1854 mL | 0.3708 mL | 0.7416 mL | 0.927 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
L-NAME hydrochloride
- 7,4'-Di-O-methylapigenin
Catalog No.:BCN5634
CAS No.:5128-44-9
- 5,7-Diacetoxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1432
CAS No.:5128-43-8
- Afzelechin 3-O-xyloside
Catalog No.:BCN7774
CAS No.:512781-45-2
- 2,6-Dimethyl-3,7-octadiene-2,6-diol
Catalog No.:BCN5633
CAS No.:51276-34-7
- Gallocatechin gallate
Catalog No.:BCN6803
CAS No.:5127-64-0
- Amsacrine
Catalog No.:BCC4309
CAS No.:51264-14-3
- N-Phthaloyl-Phe-OH
Catalog No.:BCC3016
CAS No.:5123-55-7
- Wighteone
Catalog No.:BCN5632
CAS No.:51225-30-0
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
- Alpha-Eudesmol
Catalog No.:BCC8272
CAS No.:473-16-5
- 27-Hydroxymangiferonic acid
Catalog No.:BCN4626
CAS No.:5132-66-1
- Tizanidine
Catalog No.:BCC4082
CAS No.:51322-75-9
- Aloe-emodin-glucoside
Catalog No.:BCC8130
CAS No.:29010-56-8
- Soyasaponin Bb
Catalog No.:BCN2598
CAS No.:51330-27-9
- Budesonide
Catalog No.:BCC4767
CAS No.:51333-22-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
- Taraxerone
Catalog No.:BCN5636
CAS No.:514-07-8
- Abietic acid
Catalog No.:BCN2728
CAS No.:514-10-3
Dopamine induces inhibitory effects on the circular muscle contractility of mouse distal colon via D1- and D2-like receptors.[Pubmed:28600746]
J Physiol Biochem. 2016 Aug;73(3):395-404.
Dopamine (DA) acts as gut motility modulator, via D1- and D2-like receptors, but its effective role is far from being clear. Since alterations of the dopaminergic system could lead to gastrointestinal dysfunctions, a characterization of the enteric dopaminergic system is mandatory. In this study, we investigated the role of DA and D1- and D2-like receptors in the contractility of the circular muscle of mouse distal colon by organ-bath technique. DA caused relaxation in carbachol-precontracted circular muscle strips, sensitive to domperidone, D2-like receptor antagonist, and mimicked by bromocriptine, D2-like receptor agonist. 7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH-23390), D1-like receptor antagonist, neural toxins, L-NAME (nitric oxide (NO) synthase inhibitor), 2'-deoxy-N6-methyl adenosine 3',5'-diphosphate diammonium salt (MRS 2179), purinergic P2Y1 antagonist, or adrenergic antagonists were ineffective. DA also reduced the amplitude of neurally evoked cholinergic contractions. The effect was mimicked by (+/-)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide (SKF-38393), D1-like receptor agonist and antagonized by SCH-23390, MRS 2179, or L-NAME. Western blotting analysis determined the expression of DA receptor proteins in mouse distal colon. Notably, SCH-23390 per se induced an increase in amplitude of spontaneous and neurally evoked cholinergic contractions, unaffected by neural blockers, L-NAME, MRS 2179, muscarinic, adrenergic, or D2-like receptor antagonists. Indeed, SCH-23390-induced effects were antagonized by an adenylyl cyclase blocker. In conclusion, DA inhibits colonic motility in mice via D2- and D1-like receptors, the latter reducing acetylcholine release from enteric neurons, involving nitrergic and purinergic systems. Whether constitutively active D1-like receptors, linked to adenylyl cyclase pathway, are involved in a tonic inhibitory control of colonic contractility is questioned.
Systemic Evaluation of Vascular Dysfunction by High-Resolution Sonography in an Nomega -Nitro-l-Arginine Methyl Ester Hydrochloride-Induced Mouse Model of Preeclampsia-Like Symptoms.[Pubmed:28914979]
J Ultrasound Med. 2018 Mar;37(3):657-666.
OBJECTIVES: The purpose of this study was to evaluate vascular function, including arterial resistance and endothelial function, by high-resolution sonography in an Nomega -nitro-l-arginine methyl ester hydrochloride (l-NAME)-induced mouse model of preeclampsia-like symptoms. METHODS: Pregnant mice were subcutaneously injected with a saline solution (control; n = 10) or l-NAME (n = 10) between the 7th and 18th days of gestation. The resistive index and pulsatility index (RI and PI, indicators of arterial resistance) of the uteroplacental, umbilical, femoral, and common carotid arteries and the flow-mediated dilatation (index of endothelial function) of the femoral artery were measured by high-frequency sonography in both groups. RESULTS: We noted significant increases in the RI and PI of the uteroplacental and umbilical arteries and a decrease in the flow-mediated dilatation of the femoral artery in the l-NAME group compared with the control group. We also found that the RI and PI of the uteroplacental and umbilical arteries were negatively correlated with fetal weight and crown-rump length. The results of the multivariate analysis using a logistic regression model indicated that the flow-mediated dilatation at 120 seconds was an independent diagnostic criterion for the l-NAME-induced preeclampsia-like model. A receiver operating characteristic analysis showed that flow-mediated dilatation at 120 seconds had the greatest area under the curve of 0.934, with an optimal cutoff point of 11.1%, yielding sensitivity of 100% and specificity of 84.6%. CONCLUSIONS: The PI and RI of the fetomaternal vasculature can identify fetuses in "high-risk" pregnancies, and flow-mediated dilatation is a reliable indicator for predicting preeclampsia. Assessment of vascular function by high-resolution sonography provides a useful platform for preeclampsia-related basic research with high reproducibility.
Ferulic acid alleviates symptoms of preeclampsia in rats by upregulating vascular endothelial growth factor.[Pubmed:28640960]
Clin Exp Pharmacol Physiol. 2017 Oct;44(10):1026-1031.
Preeclampsia is a complication affecting pregnant women worldwide, which leads to maternal and fetal morbidity and mortality. In this study, we evaluated the efficacy of ferulic acid (FA) on an N(omega) -nitro-L-arginine methyl ester hydrochloride (L-NAME) induced rat model of preeclampsia. L-NAME was administered to pregnant rats to induce preeclampsia. 48 rats were divided into three experimental groups (n=16 each): control group, preeclampsia group and preeclampsia with FA treatment (preeclampsia+FA). Physiological characteristics such as urine volume, total urine protein and blood pressure were assessed. Expressions levels of urinary nephrin and podocin mRNAs were analyzed by RT-PCR. Levels of renal vascular endothelial growth factor (VEGF), renal soluble fms-like tyrosine kinase-1 (sFlt-1) and serum placenta growth factor (PlGF) were also examined. Urine volume, total urine protein and blood pressure were markedly increased in preeclampsia group rats compared to control (P<.05), which were then significantly reduced in preeclampsia+FA group (P<.05). Expressions of urinary nephrin and podocin mRNAs, levels of VEGF, sFlt-1 and PlGF were also reversed in preeclampsia+FA group compared to preeclampsia rats (P<.05). We hereby report for the first time, FA alleviates preeclampsia symptoms in a rat preeclampsia model, supporting its potential value in treating preeclampsia.
Aerobic Swim Training Restores Aortic Endothelial Function by Decreasing Superoxide Levels in Spontaneously Hypertensive Rats.[Pubmed:28591344]
Clinics (Sao Paulo). 2017 May;72(5):310-316.
OBJECTIVE:: We aimed to determine whether aerobic training decreases superoxide levels, increases nitric oxide levels, and improves endothelium-dependent vasodilation in the aortas of spontaneously hypertensive rats. METHODS:: Spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) were distributed into 2 groups: sedentary (SHRsd and WKYsd, n=10 each) and swimming-trained (SHRtr, n=10 and WKYtr, n=10, respectively). The trained group participated in training sessions 5 days/week for 1 h/day with an additional work load of 4% of the animal's body weight. After a 10-week sedentary or aerobic training period, the rats were euthanized. The thoracic aortas were removed to evaluate the vasodilator response to acetylcholine (10-10 to 10-4 M) with or without preincubation with L-NG-nitro-L-arginine methyl ester hydrochloride (L-NAME; 10-4 M) in vitro. The aortic tissue was also used to assess the levels of the endothelial nitric oxide synthase and nicotinamide adenine dinucleotide oxidase subunit isoforms 1 and 4 proteins, as well as the superoxide and nitrite contents. Blood pressure was measured using a computerized tail-cuff system. RESULTS:: Aerobic training significantly increased the acetylcholine-induced maximum vasodilation observed in the SHRtr group compared with the SHRsd group (85.9+/-4.3 vs. 71.6+/-5.2%). Additionally, in the SHRtr group, superoxide levels were significantly decreased, nitric oxide bioavailability was improved, and the levels of the nicotinamide adenine dinucleotide oxidase subunit isoform 4 protein were decreased compared to the SHRsd group. Moreover, after training, the blood pressure of the SHRtr group decreased compared to the SHRsd group. Exercise training had no effect on the blood pressure of the WKYtr group. CONCLUSIONS:: In SHR, aerobic swim training decreased vascular superoxide generation by nicotinamide adenine dinucleotide oxidase subunit isoform 4 and increased nitric oxide bioavailability, thereby improving endothelial function.
Hydrogen Sulfide Mediating both Excitatory and Inhibitory Effects in a Rat Model of Meningeal Nociception and Headache Generation.[Pubmed:28769868]
Front Neurol. 2017 Jul 14;8:336.
BACKGROUND/PURPOSE: Hydrogen sulfide (H2S) is a neuromodulator acting through nitroxyl (HNO) when it reacts with nitric oxide (NO). HNO activates transient receptor potential channels of the ankyrin type 1 (TRPA1) causing release of calcitonin gene-related peptide from primary afferents. Activation of meningeal nociceptors projecting to the human spinal trigeminal nucleus (STN) may lead to headaches. In a rat model of meningeal nociception, the activity of spinal trigeminal neurons was used as read-out for the interaction between H2S and NO. METHODS: In anesthetized rats extracellular recordings from single neurons in the STN were made. Sodium sulfide (Na2S) producing H2S in the tissue and the NO donor diethylamine-NONOate (DEA-NONOate) were infused intravenously. H2S was also locally applied onto the exposed cranial dura mater or the medulla. Endogenous production of H2S was inhibited by oxamic acid, and NO production was inhibited by nitro-l-arginine methyl ester hydrochloride (l-NAME) to manipulate endogenous HNO formation. KEY RESULTS: Systemic administration of Na2S was followed either by increased ongoing activity (in 73%) or decreased activity (in 27% of units). Topical application of Na2S onto the cranial dura mater caused a short-lasting activation followed by a long-lasting decrease in activity in the majority of units (70%). Systemic administration of DEA-NONOate increased neuronal activity, subsequent infusion of Na2S added to this effect, whereas DEA-NONOate did not augment the activity after Na2S. The stimulating effect of DEA-NONOate was inhibited by oxamic acid in 75% of units, and l-NAME following Na2S administration returned the activity to baseline. CONCLUSION: Individual spinal trigeminal neurons may be activated or (less frequently) inhibited by the TRPA1 agonist HNO, presumably formed by H2S and NO in the STN, whereby endogenous H2S production seems to be rate-limiting. Activation of meningeal afferents by HNO may induce decreased spinal trigeminal activity, consistent with the elevation of the electrical threshold caused by TRPA1 activation in afferent fibers. Thus, the effects of H2S-NO-TRPA1 signaling depend on the site of action and the type of central neurons. The role of H2S-NO-TRPA1 in headache generation seems to be ambiguous.
Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system?[Pubmed:9226999]
Trends Pharmacol Sci. 1997 Jun;18(6):204-11.
It is now ten years since NO was shown to account for the biological activity of endothelium-derived relaxing factor (EDRF). It is also the tenth anniversary of the identification of L-NG monomethyl arginine (L-NMMA) as the very first inhibitor of NO biosynthesis. That EDRF and NO were one and the same sparked an explosion of interest in the biochemistry and pharmacology of NO which has yet to subside. In contrast, the first ever nitric oxide synthase (NOS) inhibitor slipped seamlessly into the literature virtually without comment at the time. Over the following decade, L-NMMA (and like NOS inhibitors) have proved invaluable as tools for probing the biological roles of NO in health and disease and, in particular, have increased our understanding of the function of NO in the nervous system. Further advances in this important area now require the development of inhibitors selective for the neuronal isoform of NOS (nNOS). Here, Philip Moore and Rachel Handy provide an up-to-date account of the literature regarding the biochemical and pharmacological characterization of NOS inhibitors with particular reference to compounds with greater selectivity for the nNOS isoform.
Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): requirement for bioactivation to the free acid, NG-nitro-L-arginine.[Pubmed:8832069]
Br J Pharmacol. 1996 Jul;118(6):1433-40.
1. The L-arginine derivatives NG-nitro-L-arginine (L-NOARG) and NG-nitro-L-arginine methyl ester (L-NAME) have been widely used to inhibit constitutive NO synthase (NOS) in different biological systems. This work was carried out to investigate whether L-NAME is a direct inhibitor of NOS or requires preceding hydrolytic bioactivation to L-NOARG for inhibition of the enzyme. 2. A bolus of L-NAME and L-NOARG (0.25 micromol) increased coronary perfusion pressure of rat isolated hearts to the same extent (21 +/- 0.8 mmHg; n = 5), but the effect developed more rapidly following addition of L-NOARG than L-NAME (mean half-time: 0.7 vs 4.2 min). The time-dependent onset of the inhibitory effect of L-NAME was paralleled by the appearance of L-NOARG in the coronary effluent. 3. Freshly dissolved L-NAME was a 50 fold less potent inhibitor of purified brain NOS (mean IC50 = 70 microM) than L-NOARG (IC50 = 1.4 microM), but the apparent inhibitory potency of L-NAME approached that of L-NOARG upon prolonged incubation at neutral or alkaline pH. H.p.l.c. analyses revealed that NOS inhibition by L-NAME closely correlated with hydrolysis of the drug to L-NOARG. 4. Freshly dissolved L-NAME contained 2% of L-NOARG and was hydrolyzed with a half-life of 365 +/- 11.2 min in buffer (pH 7.4), 207 +/- 1.7 min in human plasma, and 29 +/- 2.2 min in whole blood (n = 3 in each case). When L-NAME was preincubated in plasma or buffer, inhibition of NOS was proportional to formation of L-NOARG, but in blood the inhibition was much less than expected from the rates of L-NAME hydrolysis. This was explained by accumulation of L-NOARG in blood cells. 5. These results suggest that L-NAME represents a prodrug lacking NOS inhibitory activity unless it is hydrolyzed to L-NOARG. Bioactivation of L-NAME proceeds at moderate rates in physiological buffers, but is markedly accelerated in tissues such as blood or vascular endothelium.


