OxyphyllacinolCAS# 87657-77-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 87657-77-0 | SDF | Download SDF |
| PubChem ID | 5320349 | Appearance | Oil |
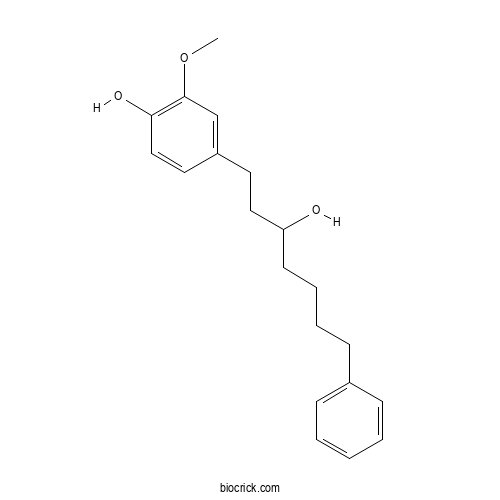
| Formula | C20H26O3 | M.Wt | 314.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(3-hydroxy-7-phenylheptyl)-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)CCC(CCCCC2=CC=CC=C2)O)O | ||
| Standard InChIKey | DHUCMVAZNHOIPY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H26O3/c1-23-20-15-17(12-14-19(20)22)11-13-18(21)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,12,14-15,18,21-22H,5-6,9-11,13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxyphyllacinol Dilution Calculator

Oxyphyllacinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1807 mL | 15.9033 mL | 31.8066 mL | 63.6132 mL | 79.5165 mL |
| 5 mM | 0.6361 mL | 3.1807 mL | 6.3613 mL | 12.7226 mL | 15.9033 mL |
| 10 mM | 0.3181 mL | 1.5903 mL | 3.1807 mL | 6.3613 mL | 7.9517 mL |
| 50 mM | 0.0636 mL | 0.3181 mL | 0.6361 mL | 1.2723 mL | 1.5903 mL |
| 100 mM | 0.0318 mL | 0.159 mL | 0.3181 mL | 0.6361 mL | 0.7952 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Epiafzelechin-(2beta-O->7,4beta->8)-ent-epicatechin
Catalog No.:BCX0076
CAS No.:135820-73-4
- Secoisolariciresinol 9,9'-diacetate
Catalog No.:BCX0075
CAS No.:848844-79-1
- Abiesinol B
Catalog No.:BCX0074
CAS No.:1190070-89-3
- Iristectorin A-6''-O-glucoside
Catalog No.:BCX0073
CAS No.:86849-71-0
- N-Methyltetrahydrocolumbamine
Catalog No.:BCX0072
CAS No.:92758-34-4
- Clinopodic acid E
Catalog No.:BCX0071
CAS No.:159736-38-6
- 11alpha-hydroxy-3,7-dioxo-5alpha-lanosta-8,24(E)-dien-26-oic acid
Catalog No.:BCX0070
CAS No.:1245703-70-1
- 1-O-Feruloylglucose
Catalog No.:BCX0069
CAS No.:64625-37-2
- Resinacein D
Catalog No.:BCX0068
CAS No.:2231061-47-3
- Mahuannin E
Catalog No.:BCX0067
CAS No.:1173887-70-1
- Methyl ganoderate D
Catalog No.:BCX0066
CAS No.:97210-12-3
- Schisphenlignan I
Catalog No.:BCX0065
CAS No.:1542234-14-9
- Abiesinol A
Catalog No.:BCX0078
CAS No.:1190070-88-2
- Germanaism C
Catalog No.:BCX0079
CAS No.:696663-52-2
- 15,26-Dihydroxylanosta-7,9(11),24-trien-3-one
Catalog No.:BCX0080
CAS No.:420781-85-7
- 11-Oxomogroside II A1
Catalog No.:BCX0081
CAS No.:942612-74-0
- Cimiracemate A
Catalog No.:BCX0082
CAS No.:478294-16-5
- Protocatechuoylcalleryanin-3-O-beta-glucopyranoside
Catalog No.:BCX0083
CAS No.:1263431-59-9
- N-Methylarmepavine
Catalog No.:BCX0084
CAS No.:74046-21-2
- Schizotenuin A
Catalog No.:BCX0085
CAS No.:144608-09-3
- Ellagic acid-4-O-beta-D-xylopyranoside
Catalog No.:BCX0086
CAS No.:139163-18-1
- Tectorigenin-7-O-beta-glucosyl-4'-O-beta-glucoside
Catalog No.:BCX0087
CAS No.:848128-32-5
- (E)-1-methoxy-2-O-(p-coumaroyl)-myo-inositol
Catalog No.:BCX0088
CAS No.:1391715-18-6
- Ellagic acid 4-O-alpha-L-arabinofuranoside
Catalog No.:BCX0089
CAS No.:358617-39-7
Targets and mechanisms of Alpinia oxyphylla Miquel fruits in treating neurodegenerative dementia.[Pubmed:36533181]
Front Aging Neurosci. 2022 Nov 30;14:1013891.
The dried and ripe fruits of Alpinia oxyphylla and ripe fruits of Alpinia oxyphylla Miquel (AO) have the effects of tonifying kidney-essence and nourishing intelligence and thus have been widely used in treating dementia. Alzheimer's disease (AD) is a typical form of neurodegenerative dementia with kidney-essence deficiency in Traditional Chinese Medicine (TCM). So far, there is a lack of systematic studies on the biological basis of tonifying kidney-essence and nourishing intelligence and the corresponding phytochemicals. In this study, we investigated the targets of AO in tonifying kidney-essence and nourishing intelligence based on the key pathophysiological processes of neurodegenerative dementia. According to ultra-high-performance liquid chromatography with triple quadrupole mass spectrometry data and Lipinski's rule of five, 49 bioactive phytochemicals from AO were identified, and 26 of them were found to target 168 key molecules in the treatment of neurodegenerative dementia. Nine phytochemicals of AO were shown to target acetylcholinesterase (ACHE), and 19 phytochemicals were shown to target butyrylcholinesterase (BCHE). A database of neurodegenerative dementia with kidney-essence deficiency involving 731 genes was constructed. Furthermore, yakuchinone B, 5-hydroxy-1,7-bis (4-hydroxy-3-methoxyphenyl) heptan-3-one (5-HYD), oxyhylladiketone, Oxyphyllacinol, butyl-beta-D-fructopyranoside, dibutyl phthalate, chrysin, yakuchinone A, rhamnetin, and rhamnocitrin were identified as the key phytochemicals from AO that regulate the pathogenesis of neurodegenerative dementia in a multitargeted manner. The approach of studying the pharmacological mechanism underlying the effects of medicinal plants and the biological basis of TCM syndrome may be helpful in studying the translation of TCM.
Microbial Transformation of Yakuchinone A and Cytotoxicity Evaluation of Its Metabolites.[Pubmed:35409351]
Int J Mol Sci. 2022 Apr 3;23(7):3992.
Yakuchinone A (1) is a bioactive diarylheptanoid isolated from the dried fruits of Alpinia oxyphylla. Microbial transformation has been recognized as an efficient method to produce new biologically active derivatives from natural products. In the present study, microbial transformation of yakuchinone A was performed with the fungus Mucor hiemalis KCTC 26779, which led to the isolation of nine new metabolites (2, 3a, 3b, and 4-9). Their structures were elucidated as (3S)-Oxyphyllacinol (2), (3S,7R)- and (3S,7S)-7-hydroxyOxyphyllacinol (3a and 3b), (3S)-Oxyphyllacinol-4'-O-beta-d-glucopyranoside (4), (3S)-4''-hydroxyOxyphyllacinol (5), (3S)-3''-hydroxyOxyphyllacinol (6), (3S)-2''-hydroxyOxyphyllacinol (7), (3S)-2''-hydroxyOxyphyllacinol-2''-O-beta-d-glucopyranoside (8), and (3S)-Oxyphyllacinol-3-O-beta-d-glucopyranoside (9) based on the comprehensive spectroscopic analyses and the application of modified Mosher's method. All compounds were evaluated for their cytotoxic activities against melanoma, as well as breast, lung, and colorectal cancer cell lines. Compound 9, which was O-glucosylated on the diarylheptanoid alkyl chain, exhibited the most selective cytotoxic activities against melanoma cell lines with the IC(50) values ranging from 6.09 to 9.74 muM, indicating that it might be considered as a possible anti-cancer lead compound.
Antiaging and Antioxidant Bioactivities of Asteraceae Plant Fractions on the Cellular Functions of the Yeast Schizosaccharomyces pombe.[Pubmed:34589709]
Adv Pharmacol Pharm Sci. 2021 Sep 18;2021:2119634.
Research on antioxidants has been gaining worldwide attention because of their essential applications for medicinal purposes. In this study, we conducted bioprospecting of six Asteraceae plants as the source of antiaging and antioxidant agents. Water and chloroform fractions from Ageratum conyzoides L., Dichrocephala integrifolia (L.f.) Kuntze, Galinsoga parviflora (Cav.), Mikania micrantha Kunth, Sphagneticola trilobata (L.) Pruski, and Synedrella nodiflora L. were collected and assayed for their in vitro antioxidant activities and potential antiaging properties using the yeast Schizosaccharomyces pombe as the model organism. Based on the in vitro assay, the water fractions of S. trilobata showed a strong antioxidant activity. Interestingly, all treatment solutions promoted the stress tolerance phenotype of S. pombe to strong H(2)O(2)-induced oxidative stress conditions. Moreover, compared with the treatments without plant extract/fraction, all extract and fraction treatments, except the chloroform fractions of A. conyzoides, promoted yeast cell longevity. Strong induction of mitochondria activity was found following the treatments with the extracts and fractions of S. nodiflora, D. integrifolia, and M. micrantha and likely mimicked the calorie restriction-induced lifespan. Interestingly, S. nodiflora water fractions significantly upregulated the mRNA transcripts of the Pap1-mediated core environmental stress response, namely, ctt1 gene in S. pombe. These data indicated that the fractions of Asteraceae plants had potential antioxidant and antiaging activities through various cellular modulations. S. nodiflora water fraction has been shown to have antioxidant and antiaging activities in S. pombe, by modulating stress tolerance response, inducing mitochondrial activity, and increasing the ctt1 gene expression. Compounds analysis identified that S. nodiflora water fraction contained some primarily compounds including Oxyphyllacinol, valine, and sugiol.
Identification of known chemicals and their metabolites from Alpinia oxyphylla fruit extract in rat plasma using liquid chromatography/tandem mass spectrometry (LC-MS/MS) with selected reaction monitoring.[Pubmed:24879483]
J Pharm Biomed Anal. 2014 Aug;97:166-77.
Alpinia oxyphylla (Yizhi) capsularfruits are commonly used in traditional medicine. Pharmacological studies have demonstrated that A. oxyphylla capsularfruits have some beneficial roles. Besides volatile oil, sesquiterpenes, diarylheptanoids and flavonoids are main bioactive constituents occurring in the Yizhi capsularfruits. The representative constituents include tectochrysin, izalpinin, chrysin, apigenin-4',7-dimethylether, kaempferide, yakuchinone A, yakuchinone B, Oxyphyllacinol and nootkatone. Their content levels in the fruit and its pharmaceutical preparations have been reported by our group. The nine phytochemicals are also the major components present in the Yizhi alcoholic extracts, which have anti-diarrheal activities. However, the fates of these constituents in the body after oral or intravenous administration remain largely unknown. In the present study, we focus on these phytochemicals albeit other concomitant compounds. The chemicals and their metabolites in rat plasma were identified using liquid chromatography/tandem mass spectrometry with selected reaction monitoring mode after orally administered Yizhi extract to rats. Rat plasma samples were treated by methanol precipitation, acidic or enzymatic hydrolysis. This target analysis study revealed that: (1) low or trace plasma levels of parent chemicals were measured after p.o. administration of Yizhi extract, Suoquan capsules and pills to rats; (2) flavonoids and diarylheptanoids formed mainly monoglucuronide metabolites; however, diglucuronide metabolites for chrysin, izalpinin and kaempferide were also detected; (3) metabolic reduction of Yizhi diarylheptanoids occurred in rats. Yakuchinone B was reduced to yakuchinone A and then to Oxyphyllacinol in a stepwise manner and subsequently glucuronidated by UDP-glucuronosyl transferase. Further research is needed to characterize the UDP-glucuronosyl transferase and reductase involved in the biotransformation of Yizhi chemicals.
Different accumulation profiles of multiple components between pericarp and seed of Alpinia oxyphylla capsular fruit as determined by UFLC-MS/MS.[Pubmed:24727421]
Molecules. 2014 Apr 10;19(4):4510-23.
Plant secondary metabolites are known to not only play a key role in the adaptation of plants to their environment, but also represent an important source of active pharmaceuticals. Alpinia oxyphylla capsular fruits, made up of seeds and pericarps, are commonly used in traditional East Asian medicines. In clinical utilization of these capsular fruits, inconsistent processing approaches (i.e., hulling pericarps or not) are employed, with the potential of leading to differential pharmacological effects. Therefore, an important question arises whether the content levels of pharmacologically active chemicals between the seeds and pericarps of A. oxyphylla are comparable. Nine secondary metabolites present in A. oxyphylla capsular fruits, including flavonoids (e.g., tectochrysin, izalpinin, chrysin, apigenin-4',7-dimethylether and kaempferide), diarylheptanoids (e.g., yakuchinone A and B and Oxyphyllacinol) and sesquiterpenes (e.g., nootkatone), were regarded as representative constituents with putative pharmacological activities. This work aimed to investigate the abundance of the nine constituents in the seeds and pericarps of A. oxyphylla. Thirteen batches of A. oxyphylla capsular fruits were gathered from different production regions. Accordingly, an ultra-fast high performance liquid chromatography/quadrupole tandem mass spectrometry (UFLC-MS/MS) method was developed and validated. We found that: (1) the nine secondary metabolites were differentially concentrated in seeds and fruit capsules; (2) nootkatone is predominantly distributed in the seeds; in contrast, the flavonoids and diarylheptanoids are mainly deposited in the capsules; and (3) the content levels of the nine secondary metabolites occurring in the capsules varied greatly among different production regions, although the nootkatone levels in the seeds were comparable among production regions. These results are helpful to evaluating and elucidating pharmacological activities of A. oxyphylla capsular fruits. Additionally, it may be of interest to elucidate the mechanisms involved in the distinct accumulation profiles of these secondary metabolites between seeds and pericarps.


