Schizotenuin ACAS# 144608-09-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144608-09-3 | SDF | Download SDF |
| PubChem ID | 46224244 | Appearance | Powder |
| Formula | C36H28O16 | M.Wt | 716.6 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
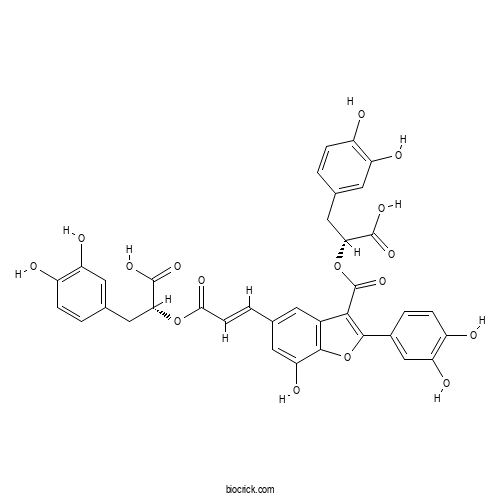
| Chemical Name | (2R)-2-[(E)-3-[3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl-2-(3,4-dihydroxyphenyl)-7-hydroxy-1-benzofuran-5-yl]prop-2-enoyl]oxy-3-(3,4-dihydroxyphenyl)propanoic acid | ||
| SMILES | C1=CC(=C(C=C1CC(C(=O)O)OC(=O)C=CC2=CC3=C(C(=C2)O)OC(=C3C(=O)OC(CC4=CC(=C(C=C4)O)O)C(=O)O)C5=CC(=C(C=C5)O)O)O)O | ||
| Standard InChIKey | KROVXXIIXUFKOO-NGJWAYPNSA-N | ||
| Standard InChI | InChI=1S/C36H28O16/c37-21-5-1-17(10-24(21)40)13-28(34(45)46)50-30(44)8-3-16-9-20-31(32(52-33(20)27(43)12-16)19-4-7-23(39)26(42)15-19)36(49)51-29(35(47)48)14-18-2-6-22(38)25(41)11-18/h1-12,15,28-29,37-43H,13-14H2,(H,45,46)(H,47,48)/b8-3+/t28-,29-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Schizotenuin A Dilution Calculator

Schizotenuin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3955 mL | 6.9774 mL | 13.9548 mL | 27.9096 mL | 34.887 mL |
| 5 mM | 0.2791 mL | 1.3955 mL | 2.791 mL | 5.5819 mL | 6.9774 mL |
| 10 mM | 0.1395 mL | 0.6977 mL | 1.3955 mL | 2.791 mL | 3.4887 mL |
| 50 mM | 0.0279 mL | 0.1395 mL | 0.2791 mL | 0.5582 mL | 0.6977 mL |
| 100 mM | 0.014 mL | 0.0698 mL | 0.1395 mL | 0.2791 mL | 0.3489 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-Methylarmepavine
Catalog No.:BCX0084
CAS No.:74046-21-2
- Protocatechuoylcalleryanin-3-O-beta-glucopyranoside
Catalog No.:BCX0083
CAS No.:1263431-59-9
- Cimiracemate A
Catalog No.:BCX0082
CAS No.:478294-16-5
- 11-Oxomogroside II A1
Catalog No.:BCX0081
CAS No.:942612-74-0
- 15,26-Dihydroxylanosta-7,9(11),24-trien-3-one
Catalog No.:BCX0080
CAS No.:420781-85-7
- Germanaism C
Catalog No.:BCX0079
CAS No.:696663-52-2
- Abiesinol A
Catalog No.:BCX0078
CAS No.:1190070-88-2
- Oxyphyllacinol
Catalog No.:BCX0077
CAS No.:87657-77-0
- Epiafzelechin-(2beta-O->7,4beta->8)-ent-epicatechin
Catalog No.:BCX0076
CAS No.:135820-73-4
- Secoisolariciresinol 9,9'-diacetate
Catalog No.:BCX0075
CAS No.:848844-79-1
- Abiesinol B
Catalog No.:BCX0074
CAS No.:1190070-89-3
- Iristectorin A-6''-O-glucoside
Catalog No.:BCX0073
CAS No.:86849-71-0
- Ellagic acid-4-O-beta-D-xylopyranoside
Catalog No.:BCX0086
CAS No.:139163-18-1
- Tectorigenin-7-O-beta-glucosyl-4'-O-beta-glucoside
Catalog No.:BCX0087
CAS No.:848128-32-5
- (E)-1-methoxy-2-O-(p-coumaroyl)-myo-inositol
Catalog No.:BCX0088
CAS No.:1391715-18-6
- Ellagic acid 4-O-alpha-L-arabinofuranoside
Catalog No.:BCX0089
CAS No.:358617-39-7
- 11-Oxomogroside IVa
Catalog No.:BCX0090
CAS No.:952481-54-8
- Tlatlancuayin
Catalog No.:BCX0091
CAS No.:3743-44-0
- Norbergenin
Catalog No.:BCX0092
CAS No.:79595-97-4
- Rhamnocitrin-3-O-neohesperoside-4'-O-glucoside
Catalog No.:BCX0093
CAS No.:446276-95-5
- Patrinia saponin H3
Catalog No.:BCX0094
CAS No.:197013-75-5
- Cimiricaside E
Catalog No.:BCX0095
CAS No.:2101977-10-8
- 24-epi-24-O-acetyl-7,8-didehydrohydroshengmanol-3-O-beta-D-xylopyranoside
Catalog No.:BCX0096
CAS No.:228251-25-0
- Raddeanoside R18
Catalog No.:BCX0097
CAS No.:781676-86-6
Phenylpropanoids on the Inhibition of beta-Amyloid Aggregation and the Movement of These Molecules through the POPC Lipid Bilayer.[Pubmed:35687701]
Langmuir. 2022 Jun 28;38(25):7775-7790.
Alzheimer's disease (AD), caused by Abeta aggregation, is a major concern in medical research. It is a neurodegenerative disorder, leading to a loss of cognitive abilities, which is still claiming the lives of many people all over the world. This poses a challenge before the scientific community to discover effective drugs which can prevent such toxic aggregation. Recent experimental findings suggest the potency of two naturally-occurring phenylpropanoids, Schizotenuin A (SCH) and Lycopic Acid B (LAB) which can effectively combat the deleterious effects of Abeta aggregation, although nothing is known about their mechanism of inhibition. In this work, we deal with an extensive computational study on the inhibitory effects of these inhibitors by using an all-atom molecular dynamics simulation to interpret the underlying mechanism of their inhibitory processes. A series of investigations is carried out while studying the various structural and conformational changes of the peptide chains in the absence and presence of inhibitors. To investigate the details of the interactions between the peptide residues and inhibitors, nonbonding energy calculations, the radial distribution function, the coordination number of water and inhibitor molecules around the peptide residues, and hydrogen-bonding interactions are calculated. The potential of mean force (PMF) is calculated to estimate aggregate formation from their free-energy profiles. It is seen that the hydrophobic core of the KLVFFAE undergoes aggregation and that these inhibitors show great promise in preventing the onset of AD in the future by preventing Abeta aggregation. Also, the translocation studies on these inhibitors through a model POPC lipid bilayer shed light on their permeation properties and biocompatibility.
Inhibitory activities of phenylpropanoids from Lycopus lucidus on amyloid aggregation related to Alzheimer's disease and type 2 diabetes.[Pubmed:32219646]
J Nat Med. 2020 Jun;74(3):579-583.
The number of patients with Alzheimer's disease (AD) and type 2 diabetes (T2D) is increasing rapidly, and thus more research has been focused on the relationship between these two age-related chronic diseases. According to the amyloid hypothesis, prevention of the aggregation of amyloid beta (Abeta) and human islet amyloid polypeptide (hIAPP) is a promising strategy for AD and T2D. In this study, thioflavin-T assay and transmission electron microscopy were performed to evaluate the inhibitory effect of three phenylpropanoids isolated from Lycopus lucidus-Schizotenuin A and lycopic acids A and B-on both Abeta and hIAPP fibrillization. All tested compounds exhibited similarly strong inhibitory activity toward amyloid aggregation. These results suggested that catechol moieties play important roles in the inhibition of amyloid plaque formation.
Studies on the preparation of bioactive lignans by oxidative coupling reaction. IV. Oxidative coupling reaction of methyl (E)-3-(3,4-dihydroxy-2-methoxyphenyl)propenoate and lipid peroxidation inhibitory effects of the produced lignans.[Pubmed:7895311]
Chem Pharm Bull (Tokyo). 1995 Jan;43(1):84-90.
The oxidative coupling reaction of the hydroxycinnamate 11 derived from daphnetin has been investigated. The reaction with silver oxide afforded, after acetylation, a dihydrobenzofuran derivative 17 and a benzodioxane derivative 16a as major products accompanied with a small amount of a bis(benzylidene)succinate 18 and a dihydronaphthalene 19, while the oxidation with iron(III) chloride gave the dihydronaphthalene derivative 20 corresponding to 19. The reaction with potassium hexacyanoferrate(III) and Na2CO3 produced, after acetylation, 16a and 19 in lower yields. The propensity for product formation in the reaction of 11 is discussed in relation to data for the reactions of hydroxycinnamate derivatives studied so far. The obtained compounds were tested for inhibitory effects on lipid peroxidation in rat brain homogenate and rat liver microsomes. In the rat brain homogenate the five compounds showed inhibitory activity more potent than that of idebenone. Compounds 17 and 20 were then tested in rat liver microsomes, and found to be more potent than Schizotenuin A and much more potent than (+/-)-alpha-tocopherol.
Studies on the preparation of bioactive lignans of oxidative coupling reaction. I. Preparation and lipid peroxidation inhibitory effect of benzofuran lignans related to schizotenuins.[Pubmed:7697764]
Chem Pharm Bull (Tokyo). 1994 Dec;42(12):2500-5.
The parent benzofuran lignan 4 of schizotenuins 1-3 and related compounds were efficiently prepared by a judicious use of the oxidative coupling reaction, and were tested for their inhibitory effects on lipid peroxidation in rat brain homogenate and rat liver microsomes. Among twelve compounds tested in rat brain homogenate, compounds 13, 14 and 16 showed prominent inhibitory activity. Compounds 13 and 16 were then tested in rat liver microsomes, and their activity was found to be more potent than Schizotenuin A (1) and much more potent than that of (+/-)-alpha-tocopherol.


