PenciclovirHSV-1 DNA synthesis inhibitor CAS# 39809-25-1 |
- Abacavir
Catalog No.:BCC1325
CAS No.:136470-78-5
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 39809-25-1 | SDF | Download SDF |
| PubChem ID | 4725 | Appearance | Powder |
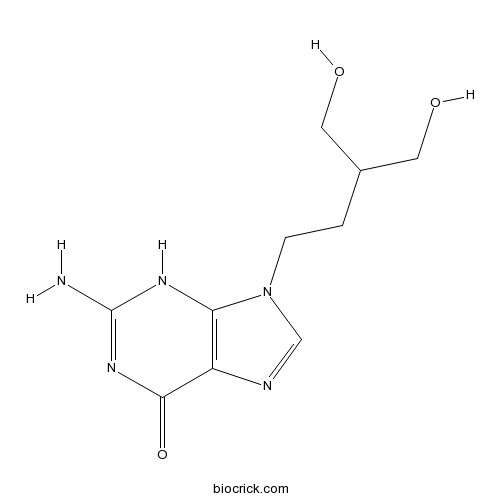
| Formula | C10H15N5O3 | M.Wt | 253.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BRL 39123; VSA 671 | ||
| Solubility | DMSO : 25 mg/mL (98.71 mM; Need ultrasonic) | ||
| Chemical Name | 2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3H-purin-6-one | ||
| SMILES | C1=NC2=C(N1CCC(CO)CO)NC(=NC2=O)N | ||
| Standard InChIKey | JNTOCHDNEULJHD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H15N5O3/c11-10-13-8-7(9(18)14-10)12-5-15(8)2-1-6(3-16)4-17/h5-6,16-17H,1-4H2,(H3,11,13,14,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Penciclovir is reported to be potent against HSV types 1 and 2 with IC50 of 0.04-1.8 μg/mL and 0.06-4.4 μg/mL, respectively.In Vitro:Penciclovir inhibits herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), varicella-zoster virus (VZV), epstein-Barr virus (EBV), human cytomegalovirus (HCMV) with IC50 of 0.04-1.8 μg/mL, 0.06-4.4 μg/mL,1.6-8 μg/mL, 1.5-3.1 μg/mL, 51 μg/mL, respectively. Penciclovir (PCV) is an acyclic guanine derivative (which is not commercially available as an oral agent), has a spectrum of activity and a mechanism of action similar to those of acyclovir (ACV)[1]. References: | |||||

Penciclovir Dilution Calculator

Penciclovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9485 mL | 19.7426 mL | 39.4851 mL | 78.9702 mL | 98.7128 mL |
| 5 mM | 0.7897 mL | 3.9485 mL | 7.897 mL | 15.794 mL | 19.7426 mL |
| 10 mM | 0.3949 mL | 1.9743 mL | 3.9485 mL | 7.897 mL | 9.8713 mL |
| 50 mM | 0.079 mL | 0.3949 mL | 0.7897 mL | 1.5794 mL | 1.9743 mL |
| 100 mM | 0.0395 mL | 0.1974 mL | 0.3949 mL | 0.7897 mL | 0.9871 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Penciclovir is an inhibitor of HSV-1 DNA synthesis [1].
Penciclovir is a guanine derivative and is used as a drug for the treatment against herpesviruses. Penciclovir has a similar spectrum of activity with acyclovir. In the plaque reduction assay, penciclovir shows potent inhibitory activities against HSV-1, HSV-2 and VZV with IC50 values of 0.4μg/ml, 1.5μg/ml and 3.1μg/ml, respectively. It also shows minimal activity against CMV. In MRC-5 cells, penciclovir inhibits the replication of HSV-1 with IC99 value of 0.4μg/ml. Moreover, penciclovir exerts persistent antiviral activity against HSV-1and HSV-2 in Vero cells. Besides that, penciclovir is found to be phosphorylated to the triphosphate in HSV-1-infected MRC-5 cells. The penciclovir triphosphate is the metabolite that actually inhibits herpesvirus replication. Penciclovir inhibits HSV-1 DNA synthesis with IC50 value of 0.16μM without effect on cellular DNA synthesis [1, 2].
References:
[1] Boyd M R, Bacon T H, Sutton D, et al. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl) guanine (BRL 39123) in cell culture. Antimicrobial agents and chemotherapy, 1987, 31(8): 1238-1242.
[2] Hodge R A, Perkins R M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrobial agents and chemotherapy, 1989, 33(2): 223-229.
- Azatadine dimaleate
Catalog No.:BCC4536
CAS No.:3978-86-7
- Boc-Tyr-OH
Catalog No.:BCC3458
CAS No.:3978-80-1
- 2-Amino-4-hydroxy-6-methylpyrimidine
Catalog No.:BCC8531
CAS No.:3977-29-5
- Bryonolol
Catalog No.:BCN2703
CAS No.:39765-50-9
- Dehydrovomifoliol
Catalog No.:BCN7562
CAS No.:39763-33-2
- 16,16-Dimethyl Prostaglandin E2
Catalog No.:BCC7843
CAS No.:39746-25-3
- H-Gln-OtBu.HCl
Catalog No.:BCC2918
CAS No.:39741-62-3
- SDZ 21009
Catalog No.:BCC7098
CAS No.:39731-05-0
- Gue 1654
Catalog No.:BCC6274
CAS No.:397290-30-1
- Daphmacropodine
Catalog No.:BCN5450
CAS No.:39729-21-0
- Catharticin
Catalog No.:BCN6850
CAS No.:39723-40-5
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
- Taibaihenryiins A
Catalog No.:BCN3281
CAS No.:398129-59-4
- Epitulipinolide diepoxide
Catalog No.:BCN5451
CAS No.:39815-40-2
- H-Ala-OiPr.HCl
Catalog No.:BCC3193
CAS No.:39825-33-7
- Amikacin disulfate
Catalog No.:BCC4622
CAS No.:39831-55-5
- Methyllinderone
Catalog No.:BCN5452
CAS No.:3984-73-4
- 19-Hydroxybufalin
Catalog No.:BCN8237
CAS No.:39844-86-5
- JNJ 5207852 dihydrochloride
Catalog No.:BCC6101
CAS No.:398473-34-2
- PHA-680632
Catalog No.:BCC2178
CAS No.:398493-79-3
- H-D-Phe(4-OMe)-OH
Catalog No.:BCC2633
CAS No.:39878-65-4
- 29-Hydroxyfriedelan-3-one
Catalog No.:BCN5453
CAS No.:39903-21-4
- 2'-Deoxycytidine hydrochloride
Catalog No.:BCC5434
CAS No.:3992-42-5
- Norglaucine hydrochloride
Catalog No.:BCN6568
CAS No.:39945-41-0
Pharmacokinetic modeling of penciclovir and BRL42359 in the plasma and tears of healthy cats to optimize dosage recommendations for oral administration of famciclovir.[Pubmed:27463546]
Am J Vet Res. 2016 Aug;77(8):833-45.
OBJECTIVES To determine, following oral administration of famciclovir, pharmacokinetic (PK) parameters for 2 of its metabolites (Penciclovir and BRL42359) in plasma and tears of healthy cats so that famciclovir dosage recommendations for the treatment of herpetic disease can be optimized. ANIMALS 7 male domestic shorthair cats. PROCEDURES In a crossover study, each of 3 doses of famciclovir (30, 40, or 90 mg/kg) was administered every 8 or 12 hours for 3 days. Six cats were randomly assigned to each dosage regimen. Plasma and tear samples were obtained at predetermined times after famciclovir administration. Pharmacokinetic parameters were determined for BRL42359 and Penciclovir by compartmental and noncompartmental methods. Pharmacokinetic-pharmacodynamic (PK-PD) indices were determined for Penciclovir and compared among all dosage regimens. RESULTS Compared with Penciclovir concentrations, BRL42359 concentrations were 5- to 11-fold greater in plasma and 4- to 7-fold greater in tears. Pharmacokinetic parameters and PK-PD indices for the 90 mg/kg regimens were superior to those for the 30 and 40 mg/kg regimens, regardless of dosing frequency. Penciclovir concentrations in tears ranged from 18% to 25% of those in plasma. Administration of 30 or 40 mg/kg every 8 hours achieved Penciclovir concentrations likely to be therapeutic in plasma but not in tears. Penciclovir concentrations likely to be therapeutic in tears were achieved only with the two 90 mg/kg regimens. CONCLUSIONS AND CLINICAL RELEVANCE In cats, famciclovir absorption is variable and its metabolism saturable. Conversion of BRL42359 to Penciclovir is rate limiting. The recommended dosage of famciclovir is 90 mg/kg every 12 hours for cats infected with feline herpesvirus.
Controlled release delivery of penciclovir via a silicone (MED-4750) polymer: kinetics of drug delivery and efficacy in preventing primary feline herpesvirus infection in culture.[Pubmed:24558980]
Virol J. 2014 Feb 22;11:34.
BACKGROUND: Herpesviruses are ubiquitous pathogens that infect and cause recurrent disease in multiple animal species. Feline herpesvirus-1 (FHV-1), a member of the alphaherpesvirus family, causes respiratory illness and conjunctivitis, and approximately 80% of domestic cats are latently infected. Oral administration of famciclovir or topical application of cidofovir has been shown in masked, placebo-controlled prospective trials to reduce clinical signs and viral shedding in experimentally inoculated cats. However, to the authors' knowledge, other drugs have not been similarly assessed or were not safe or effective. Likewise, to our knowledge, no drugs have been assessed in a placebo-controlled manner in cats with recrudescent herpetic disease. Controlled-release devices would permit long-term administration of these drugs and enhance compliance. METHODS: We therefore engineered implantable cylindrical devices made from silicone (MED-4750) impregnated with Penciclovir, for long-term, steady-state delivery of this drug. RESULTS: Our data show that these devices release Penciclovir with a burst of drug delivery until the tenth day of release, then at an average rate of 5.063 +/- 1.704 mug per day through the next 50 days with near zero-order kinetics (in comparison to MED-4750-acyclovir devices, which show the same burst kinetics and average 2.236 +/- 0.625 mug/day thereafter). Furthermore, these devices suppress primary infection of FHV-1 in a cell culture system. CONCLUSIONS: The clinical deployment of these silicone-Penciclovir devices may allow long-term treatment of FHV-1 infection with a single intervention that could last the life of the host cat.
(3)H-Penciclovir ((3)H-PCV) Uptake Assay.[Pubmed:27446976]
Bio Protoc. 2013 Sep 5;3(17).
Thymidine Kinase from human Herpes simplex virus type 1 (HSV1-TK) in combination with specific substrate prodrug nucleotide analogue ganciclovir (GCV) has been widely used as suicidal therapeutic gene for cancer gene therapy. HSV1, and its mutant (HSV1-sr39TK) with improved substrate specificity, were used as reporter genes for PET-imaging of various biological functions in small animals, by combining with radiolabeled substrates such as (18)F-FHBG and (124)I-FIAU. (3)H-Penciclovir (PCV) uptake assay is a method of choice used to determine the expression level of HSV1-TK in mammalian cells and tissues. HSV1-TK phosphorylate PCV and result in the formation of Penciclovir monophosphate, and its subsequent phopsphorylation by cellular TK lead to the formation of Penciclovir triphosphate, which is trapped selectively in cells expressing HSV-TK. (3)H-Penciclovir enables the detection of Penciclovir uptake of mammalian cells and tissues by radioactive procedures such as scintillation counting. Here we describe the protocol to carry out (3)H-Penciclovir uptakes in mammalian cells.
Indirect photochemical transformations of acyclovir and penciclovir in aquatic environments increase ecological risk.[Pubmed:26356329]
Environ Toxicol Chem. 2016 Mar;35(3):584-92.
Acyclovir and Penciclovir, 2 antiviral drugs, are increasingly detected in aquatic environments. The present study explores the natural photochemical transformation mechanisms and fate of these drugs, examining direct and indirect photochemical transformation under simulated sunlight irradiation. The 2 antiviral drugs are photostable under certain conditions but significantly degrade in the presence of chromophoric dissolved organic matter (DOM). The degradation rate associated with the drugs' indirect photochemical transformation scaled with chromophoric DOM concentration. Quenchers and sensitizers were used to identify indirect photochemical transformation mechanism. Results suggested that both pharmaceuticals could be transformed by reacting with (1)O2, (*)OH, and excited chromophoric DOM. The (1)O2 played an important role in indirect photochemical transformation. Furthermore, the reaction kinetics between their substructural molecules, guanine, isocytosine, and imidazole, with different reactive oxygen species were evaluated to determine which substrate functionalities were most susceptible to singlet oxygenation. Imidazole was identified as the reaction site for (1)O2, and preliminary (1)O2 oxidation mechanisms were further evaluated based on liquid chromatographic-tandem mass spectrometric results. Finally, aquatic ecotoxicity assessment of phototransformed solutions revealed that the degradation of acyclovir and Penciclovir may not ultimately diminish environmental risk because of either formation of more toxic intermediates than parent pharmaceuticals or some synergistic effects existing between the intermediates.


