Orotic acidCAS# 65-86-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65-86-1 | SDF | Download SDF |
| PubChem ID | 967 | Appearance | Powder |
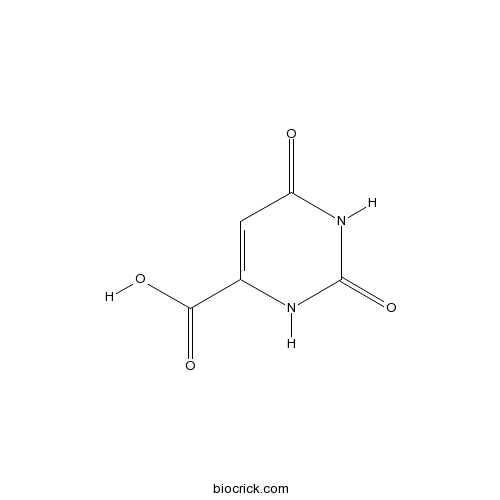
| Formula | C5H4N2O4 | M.Wt | 156.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 32 mg/mL (205.00 mM; Need ultrasonic and warming) | ||
| Chemical Name | 2,4-dioxo-1H-pyrimidine-6-carboxylic acid | ||
| SMILES | C1=C(NC(=O)NC1=O)C(=O)O | ||
| Standard InChIKey | PXQPEWDEAKTCGB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4N2O4/c8-3-1-2(4(9)10)6-5(11)7-3/h1H,(H,9,10)(H2,6,7,8,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Orotic acid Dilution Calculator

Orotic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4061 mL | 32.0307 mL | 64.0615 mL | 128.123 mL | 160.1537 mL |
| 5 mM | 1.2812 mL | 6.4061 mL | 12.8123 mL | 25.6246 mL | 32.0307 mL |
| 10 mM | 0.6406 mL | 3.2031 mL | 6.4061 mL | 12.8123 mL | 16.0154 mL |
| 50 mM | 0.1281 mL | 0.6406 mL | 1.2812 mL | 2.5625 mL | 3.2031 mL |
| 100 mM | 0.0641 mL | 0.3203 mL | 0.6406 mL | 1.2812 mL | 1.6015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Orotic acid (OA) is an intermediate in pyrimidine metabolism.
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Acridine Orange hydrochloride
Catalog No.:BCC8006
CAS No.:65-61-2
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- Gallamine Triethiodide
Catalog No.:BCC4576
CAS No.:65-29-2
- Phentolamine Mesylate
Catalog No.:BCC4353
CAS No.:65-28-1
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
- Nimorazole
Catalog No.:BCC5253
CAS No.:6506-37-2
- 6-Amino-2-methylquinoline
Catalog No.:BCC8759
CAS No.:65079-19-8
- L-152,804
Catalog No.:BCC7041
CAS No.:6508-43-6
- Sulfameter
Catalog No.:BCC4855
CAS No.:651-06-9
- Nanchangmycin
Catalog No.:BCC4970
CAS No.:65101-87-3
- NGR peptide
Catalog No.:BCC4418
CAS No.:651328-78-8
- N-Methylcorydaldine
Catalog No.:BCN3300
CAS No.:6514-05-2
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Production of orotic acid by a Klura3Delta mutant of Kluyveromyces lactis.[Pubmed:26707627]
J Biosci Bioeng. 2016 Jun;121(6):625-630.
We demonstrated that a Klura3Delta, mutant of the yeast Kluyveromyces lactis is able to produce and secrete into the growth medium considerable amounts of Orotic acid. Using yeast extract-peptone-glucose (YPD) based media we optimized production conditions in flask and bioreactor cultures. With cells grown in YPD 5% glucose medium, the best production in flask was obtained with a 1:12.5 ratio for flask: culture volume, 180 rpm, 28 degrees C and 200 mM MOPS for pH stabilization at neutral values (initial culture pH at 8.0). The best production in a 2 L bioreactor was achieved at 500 rpm with 1 vvm aeration, 28 degrees C and pH 7.0. Under these optimum conditions, similar rates of Orotic acid production were obtained and maximum concentration achieved after 96 h was 6.7 g/L in flask and bioreactor cultures. These results revealed an excellent reproducibility between both systems and provided evidence for the biotechnological potential of Klura3Delta strain to produce Orotic acid since the amounts obtained are comparable to the production in flask using a similar mutant of the industrially valuable Corynebacterium glutamicum.
Structural Properties, Order-Disorder Phenomena, and Phase Stability of Orotic Acid Crystal Forms.[Pubmed:26741914]
Mol Pharm. 2016 Mar 7;13(3):1012-29.
Orotic acid (OTA) is reported to exist in the anhydrous (AH), monohydrate (Hy1), and dimethyl sulfoxide monosolvate (SDMSO) forms. In this study we investigate the (de)hydration/desolvation behavior, aiming at an understanding of the elusive structural features of anhydrous OTA by a combination of experimental and computational techniques, namely, thermal analytical methods, gravimetric moisture (de)sorption studies, water activity measurements, X-ray powder diffraction, spectroscopy (vibrational, solid-state NMR), crystal energy landscape, and chemical shift calculations. The Hy1 is a highly stable hydrate, which dissociates above 135 degrees C and loses only a small part of the water when stored over desiccants (25 degrees C) for more than one year. In Hy1, Orotic acid and water molecules are linked by strong hydrogen bonds in nearly perfectly planar arranged stacked layers. The layers are spaced by 3.1 A and not linked via hydrogen bonds. Upon dehydration the X-ray powder diffraction and solid-state NMR peaks become broader, indicating some disorder in the anhydrous form. The Hy1 stacking reflection (122) is maintained, suggesting that the OTA molecules are still arranged in stacked layers in the dehydration product. Desolvation of SDMSO, a nonlayer structure, results in the same AH phase as observed upon dehydrating Hy1. Depending on the desolvation conditions, different levels of order-disorder of layers present in anhydrous OTA are observed, which is also suggested by the computed low energy crystal structures. These structures provide models for stacking faults as intergrowth of different layers is possible. The variability in anhydrate crystals is of practical concern as it affects the moisture dependent stability of AH with respect to hydration.
Long-term fatty liver-induced insulin resistance in orotic acid-induced nonalcoholic fatty liver rats.[Pubmed:26775542]
Biosci Biotechnol Biochem. 2016;80(4):735-43.
We investigated whether fatty liver preceded insulin resistance or vice versa using a long-term Orotic acid (OA)-induced nonalcoholic fatty liver disease (NAFLD) model without the confounding effects of obesity and hyperlipidemia and explored the role of the liver in insulin resistance. Male Wistar rats were fed with or without OA supplementation for 30, 60, and 90 days. The NAFLD group showed increased liver lipid at 30, 60, and 90 days; glucose intolerance was noted at 60 and 90 days. Furthermore, partial liver proteins and gene expressions related to upstream signaling of insulin were decreased. However, the liver glycogen content was elevated, and gluconeogenesis genes expressions were obviously decreased at 90 days. The occurrence of fatty liver preceded insulin resistance in OA-induced NAFLD without the interference of obesity and hyperlipidemia, and hepatic insulin resistance may not play a conclusive role in insulin resistance in this model.
Orotate (orotic acid): An essential and versatile molecule.[Pubmed:27906623]
Nucleosides Nucleotides Nucleic Acids. 2016 Dec;35(10-12):566-577.
Orotate (OA) is well-known as a precursor in biosynthesis of pyrimidines; in mammals it is released from the mitochondrial dihydroorotate dehydrogenase (DHODH) for conversion to UMP by the cytoplasmic UMP synthase enzyme. OA is also a normal part of the diet, being found in milk and dairy products, and it is converted to uridine for use in the pyrimidine salvage pathway predominantly in liver, kidney and erythrocytes. Early research into nutrition identified orotate as "vitamin B13," and its use as a complex with organic cations or metal ions was promulgated in body-building, and in assisting therapies of metabolic syndromes. It has recently been established that the amelioration of gout by dairy products arises from the competition of orotate and urate at the hURAT1 transporter. The Orotic aciduria that arises in children with defective UMP synthase can be rescued by oral uridine therapy, since UMP is the end-product and also a feedback inhibitor of the de novo pathway. In contrast, Miller (dysmorphology) syndrome is connected with defects in DHODH, and hence in the supply of OA, and cannot be helped by uridine. Other models of dysmorphisms are connected with enzymes early in the pyrimidine de novo pathway. We conclude that the OA molecule is itself required for the regulation of genes that are important in the development of cells, tissues and organisms.


