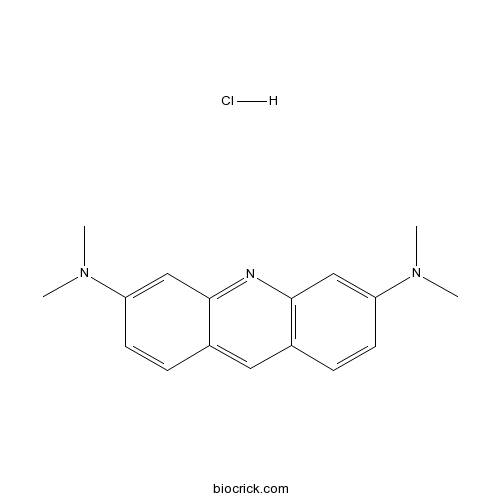
Acridine Orange hydrochlorideCAS# 65-61-2 |
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Solifenacin succinate
Catalog No.:BCC4580
CAS No.:242478-38-2
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65-61-2 | SDF | Download SDF |
| PubChem ID | 517204 | Appearance | Powder |
| Formula | C17H20ClN3 | M.Wt | 301.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 3-N,3-N,6-N,6-N-tetramethylacridine-3,6-diamine;hydrochloride | ||
| SMILES | CN(C)C1=CC2=C(C=C1)C=C3C=CC(=CC3=N2)N(C)C.Cl | ||
| Standard InChIKey | VSTHNGLPHBTRMB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H19N3.ClH/c1-19(2)14-7-5-12-9-13-6-8-15(20(3)4)11-17(13)18-16(12)10-14;/h5-11H,1-4H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell and organelle membrane permeable nucleic acid binding dye. Emits green fluorescence when bound to double stranded DNA and red fluorescence when bound to RNA or single stranded DNA. Used in cell cycle and apoptosis studies and as a lysosomal dye. |

Acridine Orange hydrochloride Dilution Calculator

Acridine Orange hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3133 mL | 16.5667 mL | 33.1334 mL | 66.2669 mL | 82.8336 mL |
| 5 mM | 0.6627 mL | 3.3133 mL | 6.6267 mL | 13.2534 mL | 16.5667 mL |
| 10 mM | 0.3313 mL | 1.6567 mL | 3.3133 mL | 6.6267 mL | 8.2834 mL |
| 50 mM | 0.0663 mL | 0.3313 mL | 0.6627 mL | 1.3253 mL | 1.6567 mL |
| 100 mM | 0.0331 mL | 0.1657 mL | 0.3313 mL | 0.6627 mL | 0.8283 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Acridine orange is a cell-permeable fluorescent dye that binds to nucleic acids, resulting in an altered spectral emission.
In Vitro:Acridine orange has been employed extensively as a cytochemical stain and has shown to stain differentially DNA and RNA, and double-restranded nucleic acids in situ. Acridine orange can either intercalate into double helical nucleic acids (green fluorescence at 530 nm), or bind electrostatically to phosphate groups of single-stranded molecules (red fluorescence at 640 nm). This unique characteristic makes acridine orange useful for cell-cycle studies[1]. Acridine orange staining of unfixed cells may be used as a simple, fast means of obtaining information on cell ploidy levels and cell cycle status from DNA measurements (green fluorescence), and cell transcriptional activity from RNA staining (red fluorescence), in human and murine cells lines, peripheral blood and bone marrow specimens from patients with leukemia and mitogenically (phytohemagglutinin) or antigenically (mixed lymphocyte culture) stimulated human peripheral blood cultures[2].
References:
[1]. McMaster GK, et al. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarosegels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835-8.
[2]. Traganos F, et al. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46-56.
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- Gallamine Triethiodide
Catalog No.:BCC4576
CAS No.:65-29-2
- Phentolamine Mesylate
Catalog No.:BCC4353
CAS No.:65-28-1
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- 7alpha-Hydroxystigmasterol
Catalog No.:BCN4194
CAS No.:64998-19-2
- Pifithrin-μ
Catalog No.:BCC2412
CAS No.:64984-31-2
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
- Nimorazole
Catalog No.:BCC5253
CAS No.:6506-37-2
- 6-Amino-2-methylquinoline
Catalog No.:BCC8759
CAS No.:65079-19-8
- L-152,804
Catalog No.:BCC7041
CAS No.:6508-43-6
- Sulfameter
Catalog No.:BCC4855
CAS No.:651-06-9
Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression.[Pubmed:24278362]
PLoS One. 2013 Nov 20;8(11):e80998.
V-ATPase is involved in the acidification of the microenvironment around/in solid tumors, such as oral squamous cell carcinoma (OSCC). V-ATPase is thought to induce tumor invasion and multi-drug resistance in several malignant tumors, and it also contributes to maintaining the intracellular pH under an acidic microenvironment by inducing proton extrusion into the extracellular medium. However, there is little information regarding the effects of V-ATPase inhibitors on OSCCs. In this study, the effects of a V-ATPase inhibitor, concanamycin A1 (CMA), on the proliferation and apoptosis of OSCC were investigated in vitro. We used four OSCC cell lines, MISK81-5, SAS, HSC-4 and SQUU-B. Acridine orange staining revealed that the red fluorescence was reduced in all of the low concentration CMA-treated OSCC cells, indicating that the acidification of vesicular organelles in the OSCCs was prevented by the treatment with low-concentration of CMA. CMA treatment induced apoptosis in MISK81-5, SAS and HSC-4 cells, but not in SQUU-B cells. The p-p38 expression was not altered in CMA-treated SQUU-B cells, but their levels were increased in the other cells. The Bax/Bcl-2 ratio in CMA-treated SQUU-B cells was dramatically decreased in comparison with that in the other cell lines treated with CMA. However, when the SQUU-B cells were treated with CMA and a histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), the SQUU-B cells became more susceptible to the CMA-induced apoptosis. SAHA treatment led to a significantly decrease in the Bcl-2 expression in CMA-treated SQUU-B cells, resulting in a dramatically increased Bax/Bcl-2 ratio in comparison with that observed in the SQUU-B cells treated with CMA alone. These findings suggest that CMA could have an anti-tumor effect on OSCCs. In addition, combination of CMA with other agents, such as SAHA, could help improve the pro-apoptotic effects of CMA even in CMA-resistant OSCC cells.
Oxidative stress induces apoptosis in embryonic cortical neurons.[Pubmed:7903353]
J Neurochem. 1994 Jan;62(1):376-9.
Glutamate-induced glutathione depletion in immature embryonic cortical neurons has been shown to lead to oxidative stress and cell death. We have used this in vitro model to investigate the mechanism(s) by which free radicals induce neuronal degeneration. We find that glutathione depletion leads to hyper-condensation and fragmentation of chromatin into spherical or irregular shapes, a morphologic signature of apoptosis. These morphologic changes are accompanied by laddering of DNA into multiple oligonucleosomal fragments and can be prevented by the antioxidants idebenone and butylated hydroxyanisole. Cell death induced by glutathione depletion can also be prevented by inhibitors of macromolecular synthesis. Taken together, these observations suggest that oxidative stress can induce apoptosis in neurons.
Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange.[Pubmed:73185]
Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835-8.
We have developed a simple and rapid system for the denaturation of nucleic acids and their subsequent analysis by gel electrophoresis. RNA and DNA are denatured in 1 M glyoxal (ethanedial) and 50% (vol/vol) dimethyl sulfoxide, at 50 degrees. The glyoxalated nucleic acids are then subjected to electrophoresis through either acrylamide or agarose gels in a 10 mM sodium phosphate buffer at pH 7.0. When glyoxalated DNA molecules of known molecular weights are used as standards, accurate molecular weights for RNA are obtained. Furthermore, we have employed the metachromatic stain acridine orange for visualization of nucleic acids in gels. This dye interacts differently with double- and single-stranded polynucleotides, fluorescing green and red, respectively. By using these techniques, native and denatured DNA and RNA molecules can be analyzed on the same slab gel.


