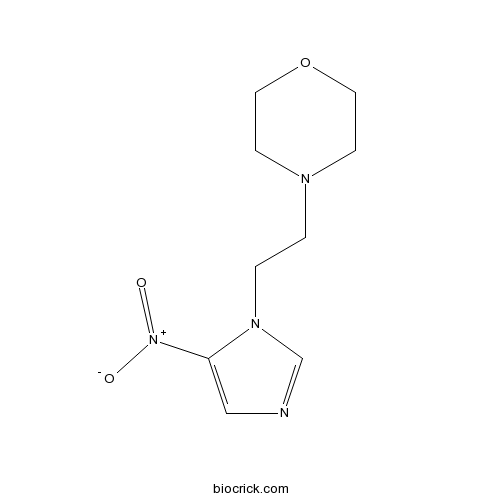
NimorazoleCAS# 6506-37-2 |
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6506-37-2 | SDF | Download SDF |
| PubChem ID | 23009 | Appearance | Powder |
| Formula | C9H14N4O3 | M.Wt | 226.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (147.33 mM; Need ultrasonic) | ||
| Chemical Name | 4-[2-(5-nitroimidazol-1-yl)ethyl]morpholine | ||
| SMILES | C1COCCN1CCN2C=NC=C2[N+](=O)[O-] | ||
| Standard InChIKey | MDJFHRLTPRPZLY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H14N4O3/c14-13(15)9-7-10-8-12(9)2-1-11-3-5-16-6-4-11/h7-8H,1-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nimorazole Dilution Calculator

Nimorazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4203 mL | 22.1014 mL | 44.2028 mL | 88.4056 mL | 110.507 mL |
| 5 mM | 0.8841 mL | 4.4203 mL | 8.8406 mL | 17.6811 mL | 22.1014 mL |
| 10 mM | 0.442 mL | 2.2101 mL | 4.4203 mL | 8.8406 mL | 11.0507 mL |
| 50 mM | 0.0884 mL | 0.442 mL | 0.8841 mL | 1.7681 mL | 2.2101 mL |
| 100 mM | 0.0442 mL | 0.221 mL | 0.442 mL | 0.8841 mL | 1.1051 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Acridine Orange hydrochloride
Catalog No.:BCC8006
CAS No.:65-61-2
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- 6-Amino-2-methylquinoline
Catalog No.:BCC8759
CAS No.:65079-19-8
- L-152,804
Catalog No.:BCC7041
CAS No.:6508-43-6
- Sulfameter
Catalog No.:BCC4855
CAS No.:651-06-9
- Nanchangmycin
Catalog No.:BCC4970
CAS No.:65101-87-3
- NGR peptide
Catalog No.:BCC4418
CAS No.:651328-78-8
- N-Methylcorydaldine
Catalog No.:BCN3300
CAS No.:6514-05-2
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
- Elastase Inhibitor, SPCK
Catalog No.:BCC1226
CAS No.:65144-34-5
- 7-Hydroxyflavanone
Catalog No.:BCN6539
CAS No.:6515-36-2
- 4'-Hydroxyflavanone
Catalog No.:BCN6548
CAS No.:6515-37-3
- (16R)-Dihydrositsirikine
Catalog No.:BCN4195
CAS No.:6519-26-2
- (16R)-E-Isositsirikine
Catalog No.:BCN4000
CAS No.:6519-27-3
A rapid and sensitive determination of hypoxic radiosensitizer agent nimorazole in rat plasma by LC-MS/MS and its application to a pharmacokinetic study.[Pubmed:25845449]
Biomed Chromatogr. 2015 Oct;29(10):1575-80.
A highly sensitive, accurate and robust LC-MS/MS method was developed and validated for determination of Nimorazole (NMZ) in rat plasma using metronidazole (MNZ) as internal standard (IS). The analyte and IS were extracted from plasma by precipitating protein with acetonitrile and were chromatographed using an Agilent Poroshell 120, EC-C18 column. The mobile phase was composed of a mixture of acetonitrile and 0.1 % formic acid (85:15 v/v). The total run time was 1.5 min and injection volume was 5 muL. Multiple reaction monitoring mode using the transitions of m/z 227.1 --> m/z 114.0 for MNZ and m/z 172.10 --> m/z 128.1 for IS were monitored on a triple quadrupole mass spectrometer, operating in positive ion mode. The calibration curve was linear in the range of 0.25-200 ng/mL (r(2) > 0.9996) and the lower limit of quantification was 0.25 ng/mL in the rat plasma samples. Recoveries of NMZ ranged between 88.05 and 95.25%. The precision (intra-day and inter-day) and accuracy of the quality control samples were 1.25-8.20% and -2.50-3.10, respectively. The analyte and IS were found to be stable during all sample storage and analysis procedures. The LC-MS/MS method described here was validated and successfully applied to pharmacokinetic study in rats.
IAEA-HypoX. A randomized multicenter study of the hypoxic radiosensitizer nimorazole concomitant with accelerated radiotherapy in head and neck squamous cell carcinoma.[Pubmed:25913070]
Radiother Oncol. 2015 Jul;116(1):15-20.
PURPOSE: To test the hypothesis that radiotherapy (RT) of head and neck squamous cell carcinoma (HNSCC) can be improved by hypoxic modification using Nimorazole (NIM) in association with accelerated fractionation. MATERIALS AND METHODS: The protocol was activated in March 2012 as an international multicenter randomized trial in patients with HNSCC. Tumors were treated to a dose of 66-70Gy, 33-35 fractions, 6 fractions per week. NIM was administered in a dose of 1.2gperm(2), 90min before the first daily RT fraction. The primary endpoint was loco-regional failure. The trial was closed prematurely by June 2014 due to poor recruitment. An associated quality assurance program was performed to ensure the consistency of RT with the protocol guidelines. RESULTS: The trial was dimensioned to include 600 patients in 3years, but only 104 patients were randomized between March 2012 and May 2014 due to the inability to involve three major centers and the insufficient recruitment rate from the other participating centers. Twenty patients from two centers had to be excluded from the analysis due to the unavailability of the follow-up data. Among the remaining 84 patients, 82 patients were evaluable (39 and 43 patients in the RT+NIM and the RT-alone arms, respectively). The treatment compliance was good with only six patients not completing the full planned RT course, and 31 patients (79%) out of 39 allocated for NIM, achieving at least 90% of the prescribed drug dose. At the time of evaluation, 40 patients had failed to achieve persistent loco-regional tumor control, and a total of 45 patients had died. The use of NIM improved the loco-regional tumor control with an 18month post-randomization cumulative failure rate of 33% versus 51% in the control arm, yielding a risk difference of 18% (CI -3% to 39%; P=0.10). The corresponding values for overall death was 43% versus 62%, yielding a risk difference of 19% (CI -3% to 42%; P=0.10). Sixteen patients, out of 55 patients analyzed for hypoxic gene expression, were classified as having more hypoxic tumors. Such patients, if treated with RT alone, had a higher loco-regional tumor failure rate as compared to the rest of the patients with known hypoxic status (P=0.05). CONCLUSION: Although the trial was incomplete and suffered from a small number of patients, the results suggested an improvement in loco-regional tumor control and overall survival in patients with advanced HNSCC given the hypoxic modifier NIM in addition to accelerated fractionation RT. However, the trial also revealed that conducting multicenter and multinational study combining drug and RT in developing countries may suffer from uncontrolled and unsolvable problems.
Photoelectron Spectra and Electronic Structures of the Radiosensitizer Nimorazole and Related Compounds.[Pubmed:26344652]
J Phys Chem A. 2015 Oct 1;119(39):9986-95.
Soft X-ray photoelectron spectroscopy has been used to investigate the radiosensitizer Nimorazole and related model compounds. We report the valence and C, N, and O 1s photoemission spectra and K-edge NEXAFS spectra of gas-phase Nimorazole, 1-methyl-5-nitroimidazole, and 4(5)-nitroimidazole in combination with theoretical calculations. The valence band and core level spectra are in agreement with theory. We determine the equilibrium populations of the two tautomers in 4(5)-nitroimidazole and find a ratio of 1:0.7 at 390 K. The NEXAFS spectra of the studied nitroimidazoles show excellent agreement with spectra of compounds available in the literature that exhibit a similar chemical environment. By comparing 1-methyl-5-nitroimidazole (single tautomer) with 4(5)-nitroimidazole, we are able to disentangle the photoemission and photoabsorption spectra and identify features due to each single tautomer.


