Gallamine TriethiodideCAS# 65-29-2 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- Rocuronium
Catalog No.:BCC1906
CAS No.:143558-00-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65-29-2 | SDF | Download SDF |
| PubChem ID | 6172 | Appearance | Powder |
| Formula | C30H60I3N3O3 | M.Wt | 891.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 30 mg/mL (33.65 mM) *"≥" means soluble, but saturation unknown. | ||
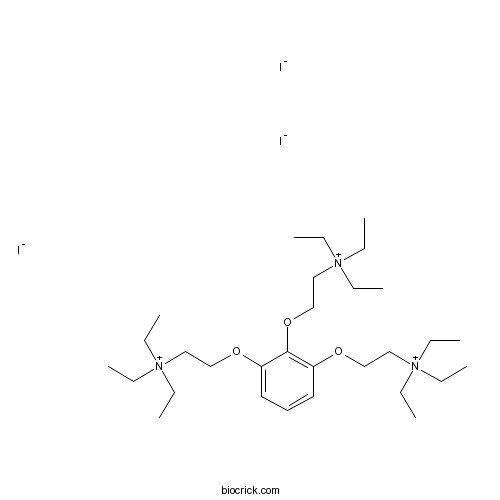
| Chemical Name | 2-[2,3-bis[2-(triethylazaniumyl)ethoxy]phenoxy]ethyl-triethylazanium;triiodide | ||
| SMILES | CC[N+](CC)(CC)CCOC1=C(C(=CC=C1)OCC[N+](CC)(CC)CC)OCC[N+](CC)(CC)CC.[I-].[I-].[I-] | ||
| Standard InChIKey | REEUVFCVXKWOFE-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/C30H60N3O3.3HI/c1-10-31(11-2,12-3)22-25-34-28-20-19-21-29(35-26-23-32(13-4,14-5)15-6)30(28)36-27-24-33(16-7,17-8)18-9;;;/h19-21H,10-18,22-27H2,1-9H3;3*1H/q+3;;;/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gallamine Triethiodide is a synthetic nondepolarizing blocking drug.
Target: mAChR
Gallamine triethiodide is a non-depolarising muscle relaxant. It acts by combining with the cholinergic receptor sites in muscle and competitively blocking the transmitter action of acetylcholine. Gallamine triethiodide has a parasympatholytic effect on the cardiac vagus nerve which causes tachycardia and occasionally hypertension. Very high doses cause histamine release. Gallamine triethiodide is commonly used to stabilize muscle contractions during surgical procedures. From Wikipedia. References: | |||||

Gallamine Triethiodide Dilution Calculator

Gallamine Triethiodide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1217 mL | 5.6083 mL | 11.2167 mL | 22.4333 mL | 28.0417 mL |
| 5 mM | 0.2243 mL | 1.1217 mL | 2.2433 mL | 4.4867 mL | 5.6083 mL |
| 10 mM | 0.1122 mL | 0.5608 mL | 1.1217 mL | 2.2433 mL | 2.8042 mL |
| 50 mM | 0.0224 mL | 0.1122 mL | 0.2243 mL | 0.4487 mL | 0.5608 mL |
| 100 mM | 0.0112 mL | 0.0561 mL | 0.1122 mL | 0.2243 mL | 0.2804 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gallamine Triethiodide is a synthetic nondepolarizing blocking drug.
- Phentolamine Mesylate
Catalog No.:BCC4353
CAS No.:65-28-1
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- 7alpha-Hydroxystigmasterol
Catalog No.:BCN4194
CAS No.:64998-19-2
- Pifithrin-μ
Catalog No.:BCC2412
CAS No.:64984-31-2
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
- H-D-Asp(OtBu)-OH
Catalog No.:BCC2899
CAS No.:64960-75-4
- Ditryptophenaline
Catalog No.:BCN7408
CAS No.:64947-43-9
- Schisantherin C
Catalog No.:BCN3621
CAS No.:64938-51-8
- Sendanolactone
Catalog No.:BCN4193
CAS No.:64929-59-5
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Acridine Orange hydrochloride
Catalog No.:BCC8006
CAS No.:65-61-2
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
Gallamine triethiodide selectively blocks voltage-gated potassium channels in Ranvier nodes.[Pubmed:11508824]
Gen Physiol Biophys. 2001 Mar;20(1):83-95.
The effects of gallamine on ionic currents in single intact Ranvier nodes of the toad Xenopus were investigated. The following fully reversible effects were observed: 1. With a test concentration of 1 mmol/l the current-voltage relation of steady-state potassium currents, IK ss exhibited a complete block of IK ss up to about V = 110 mV; with stronger depolarisations the block was incomplete. The peak sodium currents, in contrast, were not affected. 2. At the same test concentration the potassium permeability constant PK was reduced by 92% from its normal value, while the sodium permeability constant PNa decreased by only 8%. 3. Concentration-response relations of the block of PK yielded an apparent dissociation constant of 30 micromol/l and a steepness parameter of unity. Patch-clamp experiments on cloned Kv1.1, Kv1.2, Kv1.3 and Kv3.1 channels yielded apparent dissociation constants of 86, 19, >>100 and 121 micromol/l, respectively. Our findings show that gallamine is particularly well suited for separating potassium and sodium currents in axonal current ensembles. They also strongly suggest that potassium currents in Ranvier nodes of Xenopus are mainly carried by an ensemble of Kv1.1 and 1.2 channels.
Gallamine-tetraphenylborate-modified carbon paste electrode for the potentiometric determination of gallamine triethiodide (flaxedil).[Pubmed:12644209]
J Pharm Biomed Anal. 2003 Mar 26;31(4):819-26.
The construction and general performance of a novel modified carbon paste electrode for the determination of Gallamine Triethiodide have been developed. The electrode shows a stable, potentiometric response for gallamine in the concentration range 1 x 10(-3)-2 x 10(-6) M at 25 degrees C independent of pH in the range 5-8. The electrode passes a near-Nernstian cationic slope of 17.0+/-0.7 mV and lower detection limit of 1 x 10(-6) M with a fast response time of 20-45 s. Selectivity coefficients for gallamine relative to a number of interfering substances were investigated. There is a negligible interference from the studied cations, anions, and pharmaceutical excipients. The determination of gallamine in aqueous solution shows an average recovery of 99.5% and a mean relative standard deviation (RSD) of 1.4% at 100 microg/ml. The direct determination of gallamine in injection solution gave results that compare favorably with those obtained by the British pharmacopoeia method. Potentiometric titration of gallamine with sodium tetraphenylborate and phosphotungstic acid as a titrant has been monitored with the modified carbon paste electrode as an end-point indicator electrode.
The inhibitory effect of the neuromuscular blocking agent, gallamine triethiodide, on camel retina acetylcholinesterase activity.[Pubmed:9020401]
Toxicol Lett. 1997 Jan 15;90(1):45-51.
The present investigation addresses the mode of inhibition of the camel retinal acetylcholinesterase (AChE) activity by Gallamine Triethiodide, which is known to be a specific non-depolarizing neuromuscular blocking agent and polar cholinergic antagonist. This study gave the following results: it was found that gallamine (GA) reversibly inhibited the AChE activity in a concentration dependent manner, the IC50 being about 0.633 mM. The Km for the hydrolysis of acetylthiocholine iodide by AChE was found to be 0.0803 mM in the control system, and the value increased by 19-463% in the GA (0.125-1.0 mM) treated systems. The Vmax was 0.649 micromol/min per mg protein for the control as well as GA treated systems. Dixon as well as Lineweaver-Burk plots and their secondary replots indicated that the nature of the inhibition is of the reversible competitive type. The Ki value was estimated as 0.160 mM. The Ki value increased with an increase in substrate concentration. The turnover number (Kcat) and specificity constant (Ksp) were 62.1 min(-1) and 7.73 x 10(5) (M x min)(-1) in the control system while the value for one parameter (Ksp) was decreased by 25-83% in the GA (0.125-1.0 mM) treated systems.
Cathodic adsorptive stripping voltammetric determination of muscle relaxant: gallamine triethiodide (flaxedil).[Pubmed:11274856]
J Pharm Biomed Anal. 2001 Apr;25(1):31-7.
A sensitive and simple voltammetric method of analysis is developed for the determination of trace amounts of gallamine triethiode in phosphate media. This method is based on controlled adsorptive preconcentration of the relaxant onto a Hanging Mercury Drop Electrode (HMDE) whereby mercurous iodide salt(s) are formed. The technique used is Cathodic Linear Sweep Stripping Voltammetry (CLSSV). The adsorptive response was evaluated with respect to preconcentration time and potential. As little as 3 x 10(-9) mol dm(-3) i.e. 2.7 ppb flaxedil (proconcentration time 300 seconds) can be determined successfully. The application of this method was tested in the determination of flaxedil in pharmaceutical preparation (ampoules).


