Pifithrin-μInhibitor of p53 binding and anti-apoptotic CAS# 64984-31-2 |
- RITA (NSC 652287)
Catalog No.:BCC2238
CAS No.:213261-59-7
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- Pifithrin-α (PFTα)
Catalog No.:BCC2241
CAS No.:63208-82-2
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64984-31-2 | SDF | Download SDF |
| PubChem ID | 327653 | Appearance | Powder |
| Formula | C8H7NO2S | M.Wt | 181.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 303580 | ||
| Solubility | DMSO : ≥ 108 mg/mL (595.99 mM) *"≥" means soluble, but saturation unknown. | ||
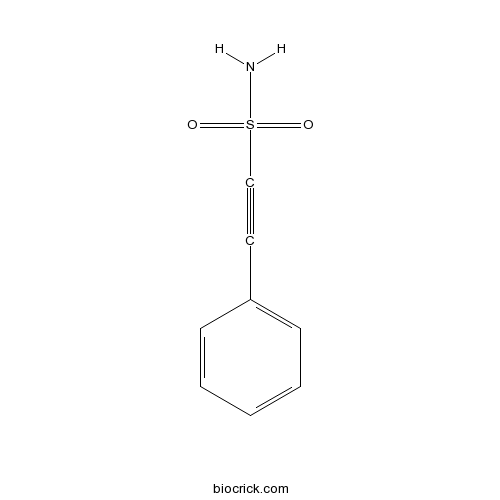
| Chemical Name | 2-phenylethynesulfonamide | ||
| SMILES | C1=CC=C(C=C1)C#CS(=O)(=O)N | ||
| Standard InChIKey | ZZUZYEMRHCMVTB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H7NO2S/c9-12(10,11)7-6-8-4-2-1-3-5-8/h1-5H,(H2,9,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibits p53 binding to mitochondria by reducing its affinity for antiapoptotic proteins Bcl-2 and Bcl-XL. Displays no effect on the transactivational or cell cycle checkpoint control function of p53. Potentially increases reprogramming efficiency of human somatic cells to induced pluripotent stem cells (iPSCs) by silencing p53. Reduces cell death induced by γ-radiation in vitro and protects mice from doses of radiation that cause lethal hematopoietic syndrome. Selectively inhibits heat shock protein 70 (HSP70) activity. |

Pifithrin-μ Dilution Calculator

Pifithrin-μ Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5185 mL | 27.5923 mL | 55.1846 mL | 110.3692 mL | 137.9615 mL |
| 5 mM | 1.1037 mL | 5.5185 mL | 11.0369 mL | 22.0738 mL | 27.5923 mL |
| 10 mM | 0.5518 mL | 2.7592 mL | 5.5185 mL | 11.0369 mL | 13.7961 mL |
| 50 mM | 0.1104 mL | 0.5518 mL | 1.1037 mL | 2.2074 mL | 2.7592 mL |
| 100 mM | 0.0552 mL | 0.2759 mL | 0.5518 mL | 1.1037 mL | 1.3796 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pifithrin-μ is a potent inhibitor of p53 binding and p53-mediated apoptosis with Kd value of 0.82 mM in vitro[1].
The p53 is encoded in humans by the TP53 gene. The molecular mass of p53 is 53 KD. The p53 has the function of regulating the cell cycle, thus, it functions as preventing cancer, a tumor suppressor. The p53 plays an important role in apoptosis, inhibition of angiogenesis and genomic stability by activating DNA repair proteins, arresting cell growth though holding the cell cycle and initiating apoptosis. p53 becomes activated in response to DNA damage, osmotic shock, oxidative stress or other myriad stressors. Activated p53 activates the expression of several genes by binding DNA including p21. p21 binds to the G1-S/CDK complexes which is an important molecules for the G1/S transition, then causes cell cycle arrest. The increasing amount of p53 may be a solution for prevention of tumors spreading or treatment of them[1].
Pifithrin-μis a cell-permeable inhibitor of p53-binding and p53-mediated apoptosis. Pifithrin-μdirectly inhibits that p53 binds to mitochondria. Pifithrin-μ also inhibits p53 binds to Bcl-2 and Bcl-xL proteins. PFT-μbinds both Bcl-xL and p53 with Kd = 0.80 mM and 0.82 mM respectively.[1] Pifithrin-μ reduces apoptosis which triggered by nutlin-3 in ML-1 cells at 25μM [2]. Pifithrin-μ also selectively inhibits heat shock protein 70 (HSP 70) activity.
Pre-treatment with Pifithrin-μcan rescue primary thymocytes from γ-irradiation or DNA damaging agents in mice.[2]
References:
[1]. Hagn F, Klein C, Demmer O, Marchenko N, Vaseva A, Moll UM, Kessler H: BclxL changes conformation upon binding to wild-type but not mutant p53 DNA binding domain. J Biol Chem 2010, 285(5):3439-3450.
[2]. Vaseva AV, Marchenko ND, Moll UM: The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle 2009, 8(11):1711-1719.
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
- H-D-Asp(OtBu)-OH
Catalog No.:BCC2899
CAS No.:64960-75-4
- Ditryptophenaline
Catalog No.:BCN7408
CAS No.:64947-43-9
- Schisantherin C
Catalog No.:BCN3621
CAS No.:64938-51-8
- Sendanolactone
Catalog No.:BCN4193
CAS No.:64929-59-5
- Schisantherin E
Catalog No.:BCN6766
CAS No.:64917-83-5
- Schisantherin D
Catalog No.:BCN7010
CAS No.:64917-82-4
- Boc-D-Trp(For)-OH
Catalog No.:BCC2598
CAS No.:64905-10-8
- BAY 61-3606 dihydrochloride
Catalog No.:BCC1407
CAS No.:648903-57-5
- trans-Norpterosin C
Catalog No.:BCN6918
CAS No.:64890-70-6
- Urapidil HCl
Catalog No.:BCC5044
CAS No.:64887-14-5
- 7alpha-Hydroxystigmasterol
Catalog No.:BCN4194
CAS No.:64998-19-2
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
- Phentolamine Mesylate
Catalog No.:BCC4353
CAS No.:65-28-1
- Gallamine Triethiodide
Catalog No.:BCC4576
CAS No.:65-29-2
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- Cytidine
Catalog No.:BCN3415
CAS No.:65-46-3
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Acridine Orange hydrochloride
Catalog No.:BCC8006
CAS No.:65-61-2
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
Pifithrin-mu is efficacious against non-small cell lung cancer via inhibition of heat shock protein 70.[Pubmed:28004121]
Oncol Rep. 2017 Jan;37(1):313-322.
Heat-shock protein (Hsp) 70, known as a pro-survival protein, is aberrantly expressed in several malignancies. The small molecule 2-phenylethyenesulfonamide (PES), also referred to as pifithrin-mu, is known as an HSP70 inhibitor, which exhibits antitumor activities in a variety of cancer cell lines. However, little is known about its effect on non-small cell lung cancer (NSCLC) cell lines. This study aimed to investigate the effect of PES on human NSCLC cell lines A549 and H460, and explore the possible underlying mechanism of action. Cell viability assay by using CCK-8 kits was performed to demonstrate that PES dose- and time-dependently inhibited proliferation of A549 and H460 cells. Wound healing assay and Transwell migration assay results indicated that PES inhibited cell migration of A549 and H460 cells. Flow cytometry results demonstrated that PES resulted in G0/G1 phase cell cycle arrest, and induced apoptosis via a caspase-dependent manner in A549 and H460 cells. Western blotting results suggested that phosphorylation of AKT and ERK was inhibited by PES treatment. In addition, death receptor 4 (DR4) and DR5 were increased by PES treatment. Overexpression of Hsp70 in A549 cells attenuated the growth inhibitory efficiency of PES. Knockdown of Hsp70 in A549 cells enhanced sensitivity of PES to cell growth inhibition, suggesting that the inhibitory effect of PES on cell proliferation is specifically through Hsp70-dependent mechanism. PES and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) exerts a potent synergistic effect on cell proliferation inhibition and induction of apoptosis in A549 and H460 cells. In a mouse xenograft model of lung cancer by A549 cells, PES treatment displayed significant inhibitory effects on tumor growth. All these findings suggest that PES shows antitumor activity against human NSCLC in vitro and in vivo, and therefore may be a promising agent for use to the treatment of NSCLC.
Novel Improved Synthesis of HSP70 Inhibitor, Pifithrin-mu. In Vitro Synergy Quantification of Pifithrin-mu Combined with Pt Drugs in Prostate and Colorectal Cancer Cells.[Pubmed:27455212]
Molecules. 2016 Jul 21;21(7). pii: molecules21070949.
We describe a novel improved approach to the synthesis of the important and well-known heat shock protein 70 inhibitor (HSP70), pifithrin-mu, with corresponding and previously unreported characterisation. The first example of a combination study comprising HSP70 inhibitor pifithrin-mu and cisplatin or oxaliplatin is reported. We have determined, using the Chou-Talalay method, (i) moderate synergistic and synergistic effects in co-treating PC-3 prostate cancer cells with pifithrin-mu and cisplatin and (ii) significant synergistic effects including strong synergism in cotreating HT29 colorectal cancer cells with oxaliplatin and pifithrin-mu.
Correction: Griffith, D.M., et al. Novel Improved Synthesis of HSP70 Inhibitor, Pifithrin-mu. In Vitro Synergy Quantification of Pifithrin-mu Combined with Pt Drugs in Prostate and Colorectal Cancer Cells. Molecules 2016, 21, 949.[Pubmed:27869678]
Molecules. 2016 Nov 17;21(11). pii: molecules21111550.
n/a.
Pifithrin-mu Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function.[Pubmed:27879267]
Cancer Res. 2017 Feb 1;77(3):742-752.
Cognitive impairment, termed chemobrain, is a common neurotoxicity associated with chemotherapy treatment, affecting an estimated 78% of patients. Prompted by the hypothesis that neuronal mitochondrial dysfunction underlies chemotherapy-induced cognitive impairment (CICI), we explored the efficacy of administering the small-molecule pifithrin (PFT)-mu, an inhibitor of mitochondrial p53 accumulation, in preventing CICI. Male C57BL/6J mice injected with cisplatin +/- PFT-mu for two 5-day cycles were assessed for cognitive function using novel object/place recognition and alternation in a Y-maze. Cisplatin impaired performance in the novel object/place recognition and Y-maze tests. PFT-mu treatment prevented CICI and associated cisplatin-induced changes in coherency of myelin basic protein fibers in the cingular cortex and loss of doublecortin(+) cells in the subventricular zone and hippocampal dentate gyrus. Mechanistically, cisplatin decreased spare respirator capacity of brain synaptosomes and caused abnormal mitochondrial morphology, which was counteracted by PFT-mu administration. Notably, increased mitochondrial p53 did not lead to cerebral caspase-3 activation or cytochrome-c release. Furthermore, PFT-mu administration did not impair the anticancer efficacy of cisplatin and radiotherapy in tumor-bearing mice. Our results supported the hypothesis that neuronal mitochondrial dysfunction induced by mitochondrial p53 accumulation is an underlying cause of CICI and that PFT-mu may offer a tractable therapeutic strategy to limit this common side-effect of many types of chemotherapy. Cancer Res; 77(3); 742-52. (c)2016 AACR.
Linking the p53 tumour suppressor pathway to somatic cell reprogramming.[Pubmed:19668186]
Nature. 2009 Aug 27;460(7259):1140-4.
Reprogramming somatic cells to induced pluripotent stem (iPS) cells has been accomplished by expressing pluripotency factors and oncogenes, but the low frequency and tendency to induce malignant transformation compromise the clinical utility of this powerful approach. We address both issues by investigating the mechanisms limiting reprogramming efficiency in somatic cells. Here we show that reprogramming factors can activate the p53 (also known as Trp53 in mice, TP53 in humans) pathway. Reducing signalling to p53 by expressing a mutated version of one of its negative regulators, by deleting or knocking down p53 or its target gene, p21 (also known as Cdkn1a), or by antagonizing reprogramming-induced apoptosis in mouse fibroblasts increases reprogramming efficiency. Notably, decreasing p53 protein levels enabled fibroblasts to give rise to iPS cells capable of generating germline-transmitting chimaeric mice using only Oct4 (also known as Pou5f1) and Sox2. Furthermore, silencing of p53 significantly increased the reprogramming efficiency of human somatic cells. These results provide insights into reprogramming mechanisms and suggest new routes to more efficient reprogramming while minimizing the use of oncogenes.
A small molecule inhibitor of inducible heat shock protein 70.[Pubmed:19818706]
Mol Cell. 2009 Oct 9;36(1):15-27.
The multifunctional, stress-inducible molecular chaperone HSP70 has important roles in aiding protein folding and maintaining protein homeostasis. HSP70 expression is elevated in many cancers, contributing to tumor cell survival and resistance to therapy. We have determined that a small molecule called 2-phenylethynesulfonamide (PES) interacts selectively with HSP70 and leads to a disruption of the association between HSP70 and several of its cochaperones and substrate proteins. Treatment of cultured tumor cells with PES promotes cell death that is associated with protein aggregation, impaired autophagy, and inhibition of lysosomal function. Moreover, this small molecule is able to suppress tumor development and enhance survival in a mouse model of Myc-induced lymphomagenesis. The data demonstrate that PES disrupts actions of HSP70 in multiple cell signaling pathways, offering an opportunity to better understand the diverse functions of this molecular chaperone and also to aid in the development of new cancer therapies.
2-Phenylacetylenesulfonamide (PAS) induces p53-independent apoptotic killing of B-chronic lymphocytic leukemia (CLL) cells.[Pubmed:19515722]
Blood. 2009 Aug 6;114(6):1217-25.
We studied the actions of 2-phenylacetylenesulfonamide (PAS) on B-chronic lymphocytic leukemia (CLL) cells. PAS (5-20 microM) initiated apoptosis within 24 hours, with maximal death at 48 hours asassessed by morphology, cleavage of poly(ADP-ribose) polymerase (PARP), caspase 3 activation, and annexin V staining. PAS treatment induced Bax proapoptotic conformational change, Bax movement from the cytosol to the mitochondria, and cytochrome c release, indicating that PAS induced apoptosis via the mitochondrial pathway. PAS induced approximately 3-fold up-regulation of proapoptotic Noxa protein and mRNA levels. In addition, Noxa was found unexpectedly to be bound to Bcl-2 in PAS-treated cells. PAS treatment of CLL cells failed to up-regulate p53, suggesting that PAS induced apoptosis independently of p53. Furthermore, PAS induced apoptosis in CLL isolates with p53 gene deletion in more than 97% of cells. Normal B lymphocytes were as sensitive to PAS-induced Noxa up-regulation and apoptosis as were CLL cells. However, both T lymphocytes and bone marrow hematopoietic progenitor cells were relatively resistant to PAS. Our data suggest that PAS may represent a novel class of drug that induces apoptosis in CLL cells independently of p53 status by a mechanism involving Noxa up-regulation.
Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation.[Pubmed:16862141]
Nat Chem Biol. 2006 Sep;2(9):474-9.
p53-dependent apoptosis contributes to the side effects of cancer treatment, and genetic or pharmacological inhibition of p53 function can increase normal tissue resistance to genotoxic stress. It has recently been shown that p53 can induce apoptosis through a mechanism that does not depend on transactivation but instead involves translocation of p53 to mitochondria. To determine the impact of this p53 activity on normal tissue radiosensitivity, we isolated a small molecule named pifithrin-mu (PFTmu, 1) that inhibits p53 binding to mitochondria by reducing its affinity to antiapoptotic proteins Bcl-xL and Bcl-2 but has no effect on p53-dependent transactivation. PFTmu has a high specificity for p53 and does not protect cells from apoptosis induced by overexpression of proapoptotic protein Bax or by treatment with dexamethasone (2). PFTmu rescues primary mouse thymocytes from p53-mediated apoptosis caused by radiation and protects mice from doses of radiation that cause lethal hematopoietic syndrome. These results indicate that selective inhibition of the mitochondrial branch of the p53 pathway is sufficient for radioprotection in vivo.


