Schisantherin DCAS# 64917-82-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64917-82-4 | SDF | Download SDF |
| PubChem ID | 163067 | Appearance | Powder |
| Formula | C29H28O9 | M.Wt | 520.53 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
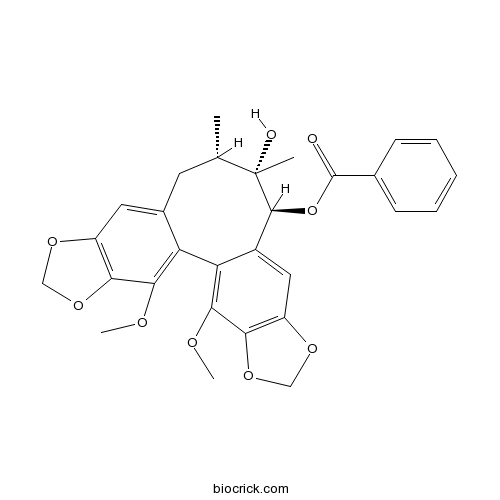
| SMILES | CC1CC2=CC3=C(C(=C2C4=C(C5=C(C=C4C(C1(C)O)OC(=O)C6=CC=CC=C6)OCO5)OC)OC)OCO3 | ||
| Standard InChIKey | PGEJVRVFUGSAJF-SSEZWIOCSA-N | ||
| Standard InChI | InChI=1S/C29H28O9/c1-15-10-17-11-19-23(36-13-34-19)25(32-3)21(17)22-18(12-20-24(26(22)33-4)37-14-35-20)27(29(15,2)31)38-28(30)16-8-6-5-7-9-16/h5-9,11-12,15,27,31H,10,13-14H2,1-4H3/t15-,27-,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Schisantherin D shows good anti-HIV activity with the EC50 value of 0.5 micrograms/mL, and the therapeutic index (TI) value of 110. |
| Targets | HIV |

Schisantherin D Dilution Calculator

Schisantherin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9211 mL | 9.6056 mL | 19.2112 mL | 38.4224 mL | 48.028 mL |
| 5 mM | 0.3842 mL | 1.9211 mL | 3.8422 mL | 7.6845 mL | 9.6056 mL |
| 10 mM | 0.1921 mL | 0.9606 mL | 1.9211 mL | 3.8422 mL | 4.8028 mL |
| 50 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7684 mL | 0.9606 mL |
| 100 mM | 0.0192 mL | 0.0961 mL | 0.1921 mL | 0.3842 mL | 0.4803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-D-Trp(For)-OH
Catalog No.:BCC2598
CAS No.:64905-10-8
- BAY 61-3606 dihydrochloride
Catalog No.:BCC1407
CAS No.:648903-57-5
- trans-Norpterosin C
Catalog No.:BCN6918
CAS No.:64890-70-6
- Urapidil HCl
Catalog No.:BCC5044
CAS No.:64887-14-5
- Rubusoside
Catalog No.:BCN2313
CAS No.:64849-39-4
- AS-605240
Catalog No.:BCC2495
CAS No.:648450-29-7
- AS-604850
Catalog No.:BCC4989
CAS No.:648449-76-7
- ophocarpine hydrobromide
Catalog No.:BCN7541
CAS No.:78003-71-1
- Vitexin-2''-O-rhamnoside
Catalog No.:BCN5025
CAS No.:64820-99-1
- Neomangiferin
Catalog No.:BCN4970
CAS No.:64809-67-2
- Heraclenol acetonide
Catalog No.:BCN4192
CAS No.:64790-68-7
- Glucagon (19-29), human
Catalog No.:BCC1012
CAS No.:64790-15-4
- Schisantherin E
Catalog No.:BCN6766
CAS No.:64917-83-5
- Sendanolactone
Catalog No.:BCN4193
CAS No.:64929-59-5
- Schisantherin C
Catalog No.:BCN3621
CAS No.:64938-51-8
- Ditryptophenaline
Catalog No.:BCN7408
CAS No.:64947-43-9
- H-D-Asp(OtBu)-OH
Catalog No.:BCC2899
CAS No.:64960-75-4
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Pifithrin-μ
Catalog No.:BCC2412
CAS No.:64984-31-2
- 7alpha-Hydroxystigmasterol
Catalog No.:BCN4194
CAS No.:64998-19-2
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
Compounds from Kadsura angustifolia with anti-HIV activity.[Pubmed:21232955]
Bioorg Med Chem Lett. 2011 Feb 1;21(3):961-5.
Four new cycloartane triterpenoids, angustific acid A (1), angustific acid B (2), angustifodilactone A (3) and angustifodilactone B (4) were isolated from the branches of Kadsura angustifolia together with six known compounds, micranoic acid B (5), nigranoic acid (6), schisandrin (7), Schisantherin D (8), interiotherin B (9), schisantherin B (10). Their structures were established on the basis of extensive spectroscopic data analyses and comparison with spectroscopic data reported. Compound 1, characterized by the presence of a C-16/C-17, C-20/C-21 conjugated diene and a C-1/C-7 ester bridge formed in rings A and B, provided a novel structural skeleton for 3,4-secocycloartane triterpenoid derivatives. In addition, the anti-HIV activities of these compounds were determined in infected C8166 cells, and it was found that angustific acid A (1) exhibited the most potent anti-HIV activity with an EC(50) value of 6.1 mug/mL and a therapeutic index of more than 32.8.
Two new lignans, interiotherins A and B, as anti-HIV principles from Kadsura interior.[Pubmed:8946749]
J Nat Prod. 1996 Nov;59(11):1066-8.
Two new lignans, interiotherins A (1) and B (2), along with two known lignans, angeloylgomisin R (3) and Schisantherin D (4), were isolated from Kadsura interior. Their structures and stereochemistries were determined from spectral data. Compounds 1 and 4 inhibit HIV replication with EC50 values of 3.1 and 0.5 micrograms/mL, respectively.
Four new lignans from Schisandra sphenanthera.[Pubmed:23945017]
J Asian Nat Prod Res. 2013 Sep;15(9):934-40.
Three new 7,8-secolignans, schisandlignans A-C (1, 2, and 4), one new dibenzocyclooctadiene lignan, schisandlignan D (5), together with nine known lignans 3',4'-dimethoxybenzoic acid (3'',4''-dimethoxyphenyl)-2-methyl-3-oxobutyl ester (3), gomisin J (6), rubrisandrin A(1b) (7), interiotherin B (8), Schisantherin D (9), ( - )-machilusin (10), ganschisandrine (11), henricine A (12), and (+)-1-hydroxy pinoresinol (13), were isolated from the rattan of Schisandra sphenanthera. Their structures were determined by analysis of 1D and 2D NMR spectroscopic data.
Chemical analysis of twelve lignans in the fruit of Schisandra sphenanthera by HPLC-PAD-MS.[Pubmed:22906629]
Phytomedicine. 2012 Oct 15;19(13):1234-41.
The fruit of S. sphenanthera, known as "Nanwuweizi", has been widely used as traditional Chinese medicine for several thousand years. However, the current determination methods are not sufficient to evaluate its quality. An accurate, sensitive and reliable high performance liquid chromatography coupled with photodiode array detection and mass spectrum (HPLC-PAD-MS) was developed for quantitative analysis of twelve lignans (schisandrol A, schisandrol B, gomisin G, schisantherin A, Schisantherin D, schisanhenol, (+)-anwulignan, deoxyschisandrin, schisandrin B, schisandrin C, 6-O-benzoylgomisin O, and interiotherin A) in the fruit of S. sphenanthera. The chromatographic conditions and extraction procedures were optimized during the study. The identity of chromatographic peaks in the sample HPLC profiles was confirmed by comparing the retention time, ultraviolet (UV) spectra and MS data with reference compounds. The validated method was successfully used to determine the twelve lignans in the samples collected from different localities in China. The hierarchical clustering analysis (HCA) and principal components analysis (PCA) were successfully applied to the data of twelve lignans from the HPLC profiles in sixteen batches of the fruit of S. sphenanthera to discriminate the samples with different sources. Moreover, the results of the loading plot of the PCA indicated that schisantherin A, (+)-anwulignan, and deoxyshisandrin were found to be the main constituents in the fruit of S. sphenanthera, and which could be chosen as the chemical markers for evaluate the quality of the fruit of S. sphenanthera. The results indicated that the developed method was readily utilized as a quality evaluation method for the fruit of S. sphenanthera.


