Picfeltarraenin IVCAS# 184288-35-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 184288-35-5 | SDF | Download SDF |
| PubChem ID | 21635749 | Appearance | Powder |
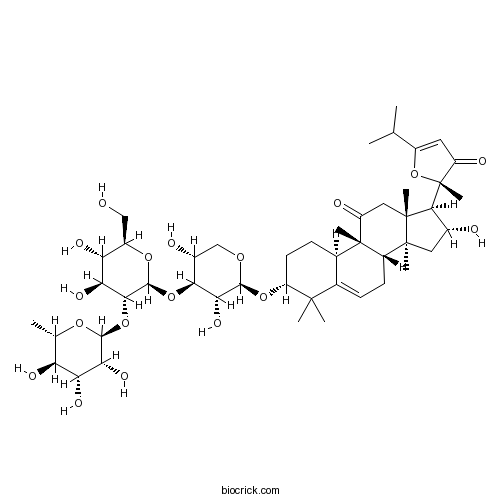
| Formula | C47H72O18 | M.Wt | 925.06 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-[(3R,8S,9R,10R,13R,14S,16R,17R)-3-[(2S,3R,4S,5R)-4-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-3,5-dihydroxyoxan-2-yl]oxy-16-hydroxy-4,4,9,13,14-pentamethyl-11-oxo-1,2,3,7,8,10,12,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]-2-methyl-5-propan-2-ylfuran-3-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2OC3C(COC(C3O)OC4CCC5C(=CCC6C5(C(=O)CC7(C6(CC(C7C8(C(=O)C=C(O8)C(C)C)C)O)C)C)C)C4(C)C)O)CO)O)O)O)O)O | ||
| Standard InChIKey | AJDSYFQMQGZVPS-TWNGIMQYSA-N | ||
| Standard InChI | InChI=1S/C47H72O18/c1-19(2)25-14-28(51)47(9,65-25)39-23(49)15-44(6)27-12-10-21-22(46(27,8)29(52)16-45(39,44)7)11-13-30(43(21,4)5)62-40-36(58)37(24(50)18-59-40)63-42-38(34(56)32(54)26(17-48)61-42)64-41-35(57)33(55)31(53)20(3)60-41/h10,14,19-20,22-24,26-27,30-42,48-50,53-58H,11-13,15-18H2,1-9H3/t20-,22+,23+,24+,26+,27-,30+,31-,32+,33+,34-,35+,36+,37-,38+,39-,40-,41-,42-,44-,45+,46-,47+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Picfeltarraenin IV is a natural product from Picria felterrae Lour. |
| Structure Identification | J. Nat. Prod., 1996, 59 (12), pp 1186–1188New Triterpenoid Saponins from Picria fel-tarrae[Reference: WebLink]Three new triterpenoid saponins, named picfeltarraenin III (1), Picfeltarraenin IV (2), and picfeltarraenin V (3), together with the known compounds picfeltarraenins IA, IB (4), and II, were isolated from whole plants of Picria fel-tarrae. The structures of 1−3 were elucidated on the basis of chemical and spectral methods. |

Picfeltarraenin IV Dilution Calculator

Picfeltarraenin IV Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.081 mL | 5.4051 mL | 10.8101 mL | 21.6202 mL | 27.0253 mL |
| 5 mM | 0.2162 mL | 1.081 mL | 2.162 mL | 4.324 mL | 5.4051 mL |
| 10 mM | 0.1081 mL | 0.5405 mL | 1.081 mL | 2.162 mL | 2.7025 mL |
| 50 mM | 0.0216 mL | 0.1081 mL | 0.2162 mL | 0.4324 mL | 0.5405 mL |
| 100 mM | 0.0108 mL | 0.0541 mL | 0.1081 mL | 0.2162 mL | 0.2703 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydromorin
Catalog No.:BCN1149
CAS No.:18422-83-8
- SR 142948
Catalog No.:BCC7323
CAS No.:184162-64-9
- GB 2a
Catalog No.:BCN7425
CAS No.:18412-96-9
- Hautriwaic acid
Catalog No.:BCN4686
CAS No.:18411-75-1
- Isoleojaponin
Catalog No.:BCN7442
CAS No.:1840966-49-5
- Calystegine B4
Catalog No.:BCN1881
CAS No.:184046-85-3
- Dimeric coniferyl acetate
Catalog No.:BCN1148
CAS No.:184046-40-0
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Ciproxifan maleate
Catalog No.:BCC4049
CAS No.:184025-19-2
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Vitisin B
Catalog No.:BCN6697
CAS No.:142449-90-9
- Cucurbitacin E
Catalog No.:BCN2300
CAS No.:18444-66-1
- Gefitinib
Catalog No.:BCN2173
CAS No.:184475-35-2
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Madecassic acid
Catalog No.:BCN1013
CAS No.:18449-41-7
- Bakkenolide B
Catalog No.:BCN7207
CAS No.:18455-98-6
- 1-Oxobakkenolide S
Catalog No.:BCN7114
CAS No.:18456-02-5
- Bakkenolide D
Catalog No.:BCN2909
CAS No.:18456-03-6
- Taxinine B
Catalog No.:BCN1150
CAS No.:18457-44-8
- 7-Deacetoxytaxinine J
Catalog No.:BCN7677
CAS No.:18457-45-9
- Taxinine J
Catalog No.:BCN6943
CAS No.:18457-46-0
- ROS 234 dioxalate
Catalog No.:BCC7245
CAS No.:184576-87-2
Bioassay- and liquid chromatography/mass spectrometry-guided acetylcholinesterase inhibitors from Picriafel-terrae.[Pubmed:24143041]
Pharmacogn Mag. 2013 Oct;9(Suppl 1):S25-31.
BACKGROUND: Picria fel-terrae is a traditional Chinese medicine. MATERIALS AND METHODS: A new approach to the search for acetylcholinesterase (AChE) inhibitors from Picria fel-terrae is presented. RESULTS: Bioassay- and LC-MS-guided fractionation of the ethyl acetate extract was from traditional Chinese medicine P.fel-terrae. Following primary extraction, the ethyl acetate extracts fraction of P.fel-terrae showed strong AChE inhibitory activities. So the sample was separated using highperformance liquid chromatography (HPLC). The effluent was split towards two identical 96-well fraction collectors, and the presence of the biologically interesting portion and chromatographic fractions could be readily detected by analyzing selected ion chromatograms through an electrophoresis-electrospray ionization mass spectrometry (ESIMS) system for accurate mass measurement. One 96-well plate was used for a bioassay (AChE-inhibitory assay) and detected the bioactivity and position of the relevant peak in the chromatogram. The positive well in the second 96-well plate was used for identification by LC-(+) ESIMS. CONCLUSION: As abovementioned, the AChE inhibitory constituents from P.fel-terrae by LC-bioassay-ESIMS were rapid identified. Liquid chromatography/ mass spectrometry (LC-MS) screening detected the presence of six active compounds, identified as picfeltarraenin IA (1), picfeltarraenin IB (2), Picfeltarraenin IV (3), picfeltarraenin X (4), picfeltarraenin XI (5), and one unknown compound. The structures were further determined by 13C NMR. The six compounds expressed stronger AChE inhibition than the known AChE inhibitorTacrine. Above all, the value of this LC-bioassay-ESIMS methodology is highlighted by the finding and structure elucidation of the active constituents from many other structural families of natural products.
A new cucurbitacin from Picria fel-terrae.[Pubmed:16864449]
J Asian Nat Prod Res. 2006 Jun;8(4):367-71.
A new cucurbitacin, picfeltarraenone II (1) as well as four known cucurbitacins, picfeltarraegenin I (2), picfeltarraenin IA (3), picfeltarraenin IB (4), and Picfeltarraenin IV (5), have been isolated and characterized from the whole plant of Picria fel-terrae. The purity of picfeltarraenin IA has been determined by TLC and HPLC.
Complement-inhibiting cucurbitacin glycosides from Picria fel-terrae.[Pubmed:9644059]
J Nat Prod. 1998 Jun 26;61(6):757-61.
Four cucurbitacin glycosides were isolated from Picriafel-terrae and identified by MS and NMR spectroscopy as picfeltarraenin IA (1), picfeltarraenin IB (2), Picfeltarraenin IV (4), and a new compound picfeltarraenin VI (3) (picfeltarraegenin I 3-O-beta-D-xylopyranoside). All four compounds acted as inhibitors on both the classical and alternative pathways of the complement system, with compound 3 exhibiting the highest inhibitory activity (IC50 29 +/- 2 microM and 21 +/- 1 microM, respectively). Compounds 1-4 showed no antiviral, antibacterial, or antifungal activities. Picfeltarraenin IA and IB were tested in an in vitro human tumor cell line panel, but displayed no cytotoxic activity.


