GB 2aCAS# 18412-96-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18412-96-9 | SDF | Download SDF |
| PubChem ID | 176988 | Appearance | Powder |
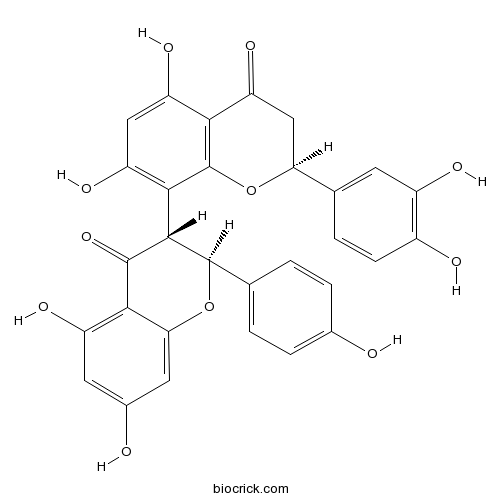
| Formula | C30H22O11 | M.Wt | 558.49 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-8-[(2S,3R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-3-yl]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=C(C1=O)C(=CC(=C2C3C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC=C(C=C5)O)O)O)C6=CC(=C(C=C6)O)O | ||
| Standard InChIKey | IMIXFUXOSFSXPC-DETITRACSA-N | ||
| Standard InChI | InChI=1S/C30H22O11/c31-14-4-1-12(2-5-14)29-27(28(39)25-18(35)8-15(32)9-23(25)41-29)26-20(37)10-19(36)24-21(38)11-22(40-30(24)26)13-3-6-16(33)17(34)7-13/h1-10,22,27,29,31-37H,11H2/t22-,27-,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. GB 2a shows antioxidant activity. 2. GB 2a biflavonoid can promote inhibition on tyrosinase activity and reduced melanin biosynthesis in B16F10 cells, which suggests great potential for medical and cosmetic uses as a depigmenting agent. 3. GB 2a has anti-inflammatory effects, it can prevent the carrageenan-induced paw oedema. |
| Targets | Tyrosinase | Immunology & Inflammation related |

GB 2a Dilution Calculator

GB 2a Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7905 mL | 8.9527 mL | 17.9054 mL | 35.8108 mL | 44.7636 mL |
| 5 mM | 0.3581 mL | 1.7905 mL | 3.5811 mL | 7.1622 mL | 8.9527 mL |
| 10 mM | 0.1791 mL | 0.8953 mL | 1.7905 mL | 3.5811 mL | 4.4764 mL |
| 50 mM | 0.0358 mL | 0.1791 mL | 0.3581 mL | 0.7162 mL | 0.8953 mL |
| 100 mM | 0.0179 mL | 0.0895 mL | 0.1791 mL | 0.3581 mL | 0.4476 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hautriwaic acid
Catalog No.:BCN4686
CAS No.:18411-75-1
- Isoleojaponin
Catalog No.:BCN7442
CAS No.:1840966-49-5
- Calystegine B4
Catalog No.:BCN1881
CAS No.:184046-85-3
- Dimeric coniferyl acetate
Catalog No.:BCN1148
CAS No.:184046-40-0
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Ciproxifan maleate
Catalog No.:BCC4049
CAS No.:184025-19-2
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- MRS 1220
Catalog No.:BCC6972
CAS No.:183721-15-5
- 1,2,3,4,5,6-Hexabromocyclohexane
Catalog No.:BCC2437
CAS No.:1837-91-8
- SR 142948
Catalog No.:BCC7323
CAS No.:184162-64-9
- Dihydromorin
Catalog No.:BCN1149
CAS No.:18422-83-8
- Picfeltarraenin IV
Catalog No.:BCN2852
CAS No.:184288-35-5
- Vitisin B
Catalog No.:BCN6697
CAS No.:142449-90-9
- Cucurbitacin E
Catalog No.:BCN2300
CAS No.:18444-66-1
- Gefitinib
Catalog No.:BCN2173
CAS No.:184475-35-2
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Madecassic acid
Catalog No.:BCN1013
CAS No.:18449-41-7
- Bakkenolide B
Catalog No.:BCN7207
CAS No.:18455-98-6
- 1-Oxobakkenolide S
Catalog No.:BCN7114
CAS No.:18456-02-5
- Bakkenolide D
Catalog No.:BCN2909
CAS No.:18456-03-6
- Taxinine B
Catalog No.:BCN1150
CAS No.:18457-44-8
Anti-inflammatory effects of hydroalcoholic extract and two biflavonoids from Garcinia gardneriana leaves in mouse paw oedema.[Pubmed:18555627]
J Ethnopharmacol. 2008 Aug 13;118(3):405-11.
Garcinia gardneriana (Planch. & Triana) Zappi (Clusiaceae) is widely distributed in Brazil and used in folk medicine to treat inflammation, pain, and urinary tract and other infections. However, very few studies have analyzed these therapeutic effects. In this study, the anti-inflammatory effects of the hydroalcoholic extracts from Garcinia gardneriana (HEGG) and some of its isolated biflavonoids were evaluated. The results showed that HEGG from the leaves, bark and seeds reduced carrageenan-induced mouse paw inflammation, in addition to diminishing the myeloperoxidase activity in the stimulated tissues. The reduction of neutrophil infiltration by treatment with the HEGG from leaves was confirmed by histology. The leaf extract also reduced the paw oedema evoked by bradykinin, histamine, prostaglandin E2 and 12-O-tetradecanoylphorbol acetate. However, it partially decreased substance P and compound 48/80-caused paw oedema, without any influence on the arachidonic acid-induced oedema. Both of the isolated compounds, fukugetin and GB-2a, prevented the carrageenan-induced paw oedema. In conclusion, this study showed important anti-inflammatory effects of HEGG through its interaction with different intracellular signaling pathways, without interfering with the formation of arachidonic acid (AA) metabolites. These characteristics, in addition to the wide distribution and culturing ease of the plant, confirm its popular use and highlight its promise in the development of new anti-inflammatory drugs.
Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis.[Pubmed:26297636]
J Ethnopharmacol. 2015 Nov 4;174:224-9.
ETHNOPHARMACOLOGY RELEVANCE: GB-2a is a I3-naringenin-II8-eriodictyol compound isolated from Garcinia gardneriana (Planchon & Triana) Zappi, a plant used in folk medicine for the treatment of skin disorders. AIM OF STUDY: In the search for new depigmenting agents, this study was carried out to investigate the in vitro effects of GB-2a isolated from G. gardneriana (Planchon & Triana) Zappi in B16F10 melanoma cells. MATERIALS AND METHODS: The effects of GB-2a were evaluated through determination of melanin biosynthesis in B16F10 melanoma cells in comparison with the reference drug kojic acid (500microM). In parallel, the GB-2a effect was assessed in a cell viability assay. Mushroom tyrosinase activity assays were conducted to verify the effect of this enzyme. In order to ascertain the nature of enzyme inhibition on tyrosinase, kinetics analysis of the GB-2a was performed with L-tyrosine and L-3,4-dihydroxyphenylalanine (L-DOPA) substrates. RESULTS: The results showed that GB-2a biflavonoid significantly inhibited the melanin content, without reducing cell viability. GB-2a also showed a strong antityrosinase activity in the mushroom tyrosinase assay. GB-2a inhibited the tyrosinase activity, exerting a mixed inhibition. For the L-tyrosine substrate the inhibition was in non-competitive mode and for L-DOPA it was in uncompetitive mode. CONCLUSION: GB-2a biflavonoid promoted inhibition on tyrosinase activity and reduced melanin biosynthesis in B16F10 cells, which suggests great potential for medical and cosmetic uses as a depigmenting agent.
Using Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry-Based Chemometrics for the Identification of Anti-angiogenic Biflavonoids from Edible Garcinia Species.[Pubmed:28926234]
J Agric Food Chem. 2017 Sep 27;65(38):8348-8355.
Garcinia xanthochymus fruits are edible and also used in traditional medicine. Our previous work showed that the isolated natural products from G. xanthochymus fruits have displayed antioxidant activity and cytotoxicity in the colon cancer cells. In this study, we developed a strategy to correlate a zebrafish angiogenesis assay with ultra-performance liquid chromatography quadrupole time of flight mass spectrometry-based chemometric analysis to identify potential anti-angiogenic activity compounds from G. xanthochymus fruits. Primary bioactivity results showed that the methanolic extracts from aril and pericarp but not from seed have significant inhibitory effects on the growth of subintestinal vessels (SIVs) in zebrafish embryos. A total of 13 markers, including benzophenones and biflavonoids, were predicted by untargeted principal component analysis and orthogonal partial least squares discriminate analysis, which were tentatively identified as priority markers for the bioactivity related in aril and pericarp. Amentoflavone, a biflavonoid, has been found to significantly inhibit the growth of SIVs at 10 and 20 muM and downregulate the expressions of Angpt2 and Tie2 genes of zebrafish embryos. Furthermore, seven biflavonoids, volkensiflavone, fukugetin, fukugeside, GB 1a, GB 1a glucoside, GB 2a, and GB 2a glucoside, isolated from Garcinia species were evaluated for their structure-activity relationship using the zebrafish model. Only fukugetin, which was previously shown to be anticancer, was active in inhibiting the SIV growth. In this report, both amentoflavone and fukugetin, for the first time, displayed anti-angiogenic effects on zebrafish, thus demonstrating an effective and rapid strategy to identify natural products for anti-angiogenesis activity.
Polyphenols from Allanblackia floribunda seeds: Identification, quantification and antioxidant activity.[Pubmed:28041556]
Food Chem. 2017 May 1;222:35-42.
Oil rich seeds of Allanblackia floribunda, a tree from tropical Africa, have traditionally been used in food preparation. Furthermore, the therapeutic properties of various parts of this tree have long been exploited in traditional medicine. As both food and pharmaceutical industries show growing interest in tropical tree crops, this study aimed to investigate whether A. floribunda seeds could also be used as a source of potentially bioactive compounds. The polyphenol profile revealed six predominant compounds which were identified by HPLC-PDA-ESI/MS(n) as the biflavonoids morelloflavone, Gb-2a and volkensiflavone and their respective glucosides. A range of less abundant flavones, flavonols and flavan-3-ols was also detected. All six major compounds showed antioxidant activity, with the activity of morelloflavone, its glucoside and Gb-2a-glucoside comparable with that of ascorbic acid. The main compounds accounted for approximately 10% of dry weight, making the seeds used for oil production a rich source of biflavonoids as a by-product.


