DihydromorinCAS# 18422-83-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18422-83-8 | SDF | Download SDF |
| PubChem ID | 5458714 | Appearance | Powder |
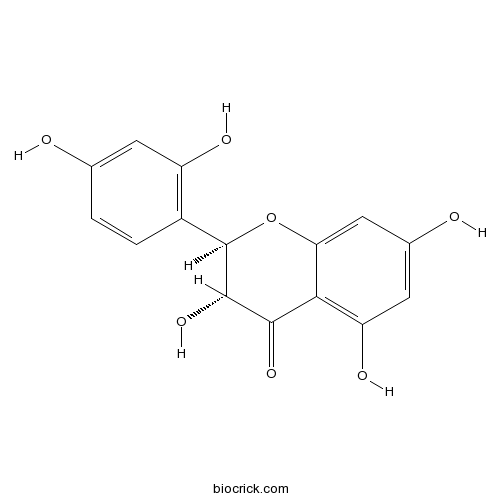
| Formula | C15H12O7 | M.Wt | 304.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | C1=CC(=C(C=C1O)O)C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O | ||
| Standard InChIKey | QIWOFDHUQPJCJF-LSDHHAIUSA-N | ||
| Standard InChI | InChI=1S/C15H12O7/c16-6-1-2-8(9(18)3-6)15-14(21)13(20)12-10(19)4-7(17)5-11(12)22-15/h1-5,14-19,21H/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | trans-Dihydromorin is an effective hypopigmenting agent in normal skin cells, hypopigmenting agents effective in melanoma system may not be effective on normal melanocytes, indicating that a non-tumor melanocyte system is more suitable for the screening of hypopigmenting agents. Dihydromorin shows strong mushroom inhibitory activity with IC50 values lower than 50 microM, more potent than (IC50 = 71.6 microM), it may be as an antibrowning agent in food systems. |
| Targets | TRP-1 | TRP-2 |
| In vitro | Oxyresveratrol and trans -dihydromorin from the twigs of Cudrania tricuspidata as hypopigmenting agents against melanogenesis[Reference: WebLink]J. Funct. Foods, 2015,13:375-83.The depigmenting effects of oxyresveratrol and trans- Dihydromorin were investigated in b16F10 cell line and its synergetic melan-a cells, as well as in 3-dimensional skin models.
Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent.[Pubmed: 18683821]Mol. Nutr. Food Res., 2008,52(12):1530-8.A new furanoflavone, 7-(2,4-dihydroxyphenyl)-4-hydroxy-2-(2-hydroxy propan-2-yl)-2, 3-dihydrofuro(3, 2-g)chromen-5-one (artocarpfuranol, 1), together with 14 known compounds, Dihydromorin (2), steppogenin (3), norartocarpetin (4), artocarpanone (5), artocarpesin (6), artocarpin (7), cycloartocarpin (8), cycloartocarpesin (9), artocarpetin (10), brosimone I (11), cudraflavone B (12), carpachromene (13), isoartocarpesin (14), and cyanomaclurin (15) were isolated from the wood of Artocarpus heterophyllus. |
| Structure Identification | J. Nat. Prod., 2002, 65 (2), pp 163–169Constituents of the Bark and Twigs of Artocarpus dadah with Cyclooxygenase Inhibitory Activity[Reference: WebLink]Fractionation of an ethyl acetate-soluble extract of the bark of Artocarpus dadah has led to the isolation of three new prenylated stilbenoid derivatives, 3-(γ,γ-dimethylallyl)resveratrol (1), 5-(γ,γ-dimethylallyl)oxyresveratrol (2), 3-(2,3-dihydroxy-3-methylbutyl)resveratrol (3), and a new benzofuran derivative, 3-(γ,γ-dimethylpropenyl)moracin M (4), along with six known compounds, oxyresveratrol, (+)-catechin, afzelechin-3-O-α-l-rhamnopyranoside, (−)-epiafzelechin, Dihydromorin, and epiafzelechin-(4β→8)-epicatechin. |

Dihydromorin Dilution Calculator

Dihydromorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2862 mL | 16.4312 mL | 32.8623 mL | 65.7246 mL | 82.1558 mL |
| 5 mM | 0.6572 mL | 3.2862 mL | 6.5725 mL | 13.1449 mL | 16.4312 mL |
| 10 mM | 0.3286 mL | 1.6431 mL | 3.2862 mL | 6.5725 mL | 8.2156 mL |
| 50 mM | 0.0657 mL | 0.3286 mL | 0.6572 mL | 1.3145 mL | 1.6431 mL |
| 100 mM | 0.0329 mL | 0.1643 mL | 0.3286 mL | 0.6572 mL | 0.8216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SR 142948
Catalog No.:BCC7323
CAS No.:184162-64-9
- GB 2a
Catalog No.:BCN7425
CAS No.:18412-96-9
- Hautriwaic acid
Catalog No.:BCN4686
CAS No.:18411-75-1
- Isoleojaponin
Catalog No.:BCN7442
CAS No.:1840966-49-5
- Calystegine B4
Catalog No.:BCN1881
CAS No.:184046-85-3
- Dimeric coniferyl acetate
Catalog No.:BCN1148
CAS No.:184046-40-0
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Ciproxifan maleate
Catalog No.:BCC4049
CAS No.:184025-19-2
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Picfeltarraenin IV
Catalog No.:BCN2852
CAS No.:184288-35-5
- Vitisin B
Catalog No.:BCN6697
CAS No.:142449-90-9
- Cucurbitacin E
Catalog No.:BCN2300
CAS No.:18444-66-1
- Gefitinib
Catalog No.:BCN2173
CAS No.:184475-35-2
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Madecassic acid
Catalog No.:BCN1013
CAS No.:18449-41-7
- Bakkenolide B
Catalog No.:BCN7207
CAS No.:18455-98-6
- 1-Oxobakkenolide S
Catalog No.:BCN7114
CAS No.:18456-02-5
- Bakkenolide D
Catalog No.:BCN2909
CAS No.:18456-03-6
- Taxinine B
Catalog No.:BCN1150
CAS No.:18457-44-8
- 7-Deacetoxytaxinine J
Catalog No.:BCN7677
CAS No.:18457-45-9
- Taxinine J
Catalog No.:BCN6943
CAS No.:18457-46-0
Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent.[Pubmed:18683821]
Mol Nutr Food Res. 2008 Dec;52(12):1530-8.
A new furanoflavone, 7-(2,4-dihydroxyphenyl)-4-hydroxy-2-(2-hydroxy propan-2-yl)-2, 3-dihydrofuro(3, 2-g)chromen-5-one (artocarpfuranol, 1), together with 14 known compounds, Dihydromorin (2), steppogenin (3), norartocarpetin (4), artocarpanone (5), artocarpesin (6), artocarpin (7), cycloartocarpin (8), cycloartocarpesin (9), artocarpetin (10), brosimone I (11), cudraflavone B (12), carpachromene (13), isoartocarpesin (14), and cyanomaclurin (15) were isolated from the wood of Artocarpus heterophyllus. Their structures were identified by interpretation of MS,( 1)H-NMR,( 13)C-NMR, HMQC, and HMBC spectroscopic data. Among them, compounds 1-6 and 14 showed strong mushroom tyrosinase inhibitory activity with IC(50) values lower than 50 microM, more potent than kojic acid (IC(50) = 71.6 microM), a well-known tyrosinase inhibitor. In addition, extract of A. heterophyllus was evaluated for its antibrowning effect on fresh-cut apple slices. It was discovered that fresh-cut apple slices treated by dipping in solution of 0.03 or 0.05% of A. heterophyllus extract with 0.5% ascorbic acid did not undergo any substantial browning reaction after storage at room temperature for 24 h. The antibrowning effect was significantly better than samples treated with the extract (0.03 or 0.05%) or ascorbic acid (0.5%) alone. The results provide preliminary evidence supporting the potential of this natural extract as antibrowning agent in food systems.


